Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury
- PMID: 26830766
- PMCID: PMC4735814
- DOI: 10.1038/srep20287
Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury
Abstract
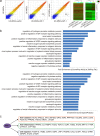
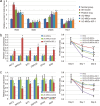
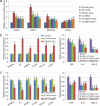
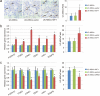
The therapeutic action of umbilical cord-derived mesenchymal stem cells (UC-MSCs) against acute kidney injury (AKI) has been demonstrated by several groups. However, how to further enhance the renoprotective effect of UC-MSCs and improve the therapy effect, are still unclear. In this study, we mainly investigated whether insulin-like growth factor-1 (IGF-1)-modified UC-MSCs hold an enhanced protective effect on gentamicin-induced AKI in vivo. Our results indicated that the IGF-1 overexpression could enhance the therapeutic action of human UC-MSCs, and the AKI rats treated with IGF-1-overexpressed UC-MSCs (UC-MSCs-IGF-1) showed better recovery of biochemical variables in serum or urine associated with renal function, histological injury and renal apoptosis, compared with AKI rats treated with normal UC-MSCs. RNA microarray analysis indicated that some key genes in the signal pathways associated with anti-oxidation, anti-inflammatory, and cell migratory capacity were up-regulated in UC-MSCs-IGF-1, and the results were further confirmed with qPCR. Furthermore, a series of detection in vitro and in vivo indicated that the UC-MSCs-IGF-1 hold better anti-oxidation, anti-inflammatory, and cell migratory capacity for IGF-1 overexpression. Thus, our study indicated that enhancement of UC-MSCs bioactivities with IGF-1 overexpression could increase the UC-MSCs therapeutic potential and further developed a new therapeutic strategy for the treatment of AKI.
Figures
Similar articles
-
Renoprotective effect of human umbilical cord-derived mesenchymal stem cells in immunodeficient mice suffering from acute kidney injury.PLoS One. 2012;7(9):e46504. doi: 10.1371/journal.pone.0046504. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23029541 Free PMC article.
-
Effects of IGF-1 on neural differentiation of human umbilical cord derived mesenchymal stem cells.Life Sci. 2016 Apr 15;151:93-101. doi: 10.1016/j.lfs.2016.03.001. Epub 2016 Mar 2. Life Sci. 2016. PMID: 26946309
-
Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro.Stem Cell Res Ther. 2013 Apr 25;4(2):34. doi: 10.1186/scrt194. Stem Cell Res Ther. 2013. PMID: 23618405 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Therapeutic Strategy for Acute Kidney Injury.Front Immunol. 2021 Jun 3;12:684496. doi: 10.3389/fimmu.2021.684496. eCollection 2021. Front Immunol. 2021. PMID: 34149726 Free PMC article. Review.
-
The application of umbilical cord-derived MSCs in cardiovascular diseases.J Cell Mol Med. 2021 Sep;25(17):8103-8114. doi: 10.1111/jcmm.16830. Epub 2021 Aug 11. J Cell Mol Med. 2021. PMID: 34378345 Free PMC article. Review.
Cited by
-
Interleukin-22 Promotes Osteosarcoma Cell Proliferation and Invasion via STAT3 Activation.Med Sci Monit. 2018 Nov 1;24:7802-7808. doi: 10.12659/MSM.910962. Med Sci Monit. 2018. PMID: 30381753 Free PMC article.
-
Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies.Stem Cell Res Ther. 2023 Jun 7;14(1):155. doi: 10.1186/s13287-023-03374-9. Stem Cell Res Ther. 2023. PMID: 37287066 Free PMC article. Review.
-
Low Insulin-Like Growth Factor-1 Level in Obesity Nephropathy: A New Risk Factor?PLoS One. 2016 May 3;11(5):e0154451. doi: 10.1371/journal.pone.0154451. eCollection 2016. PLoS One. 2016. PMID: 27138941 Free PMC article.
-
Investigation of the role of cullin 4A overexpression in human liver cancer.Mol Med Rep. 2018 Sep;18(3):2531-2540. doi: 10.3892/mmr.2018.9233. Epub 2018 Jun 29. Mol Med Rep. 2018. PMID: 30015884 Free PMC article.
-
Icariin alleviates osteoarthritis by regulating autophagy of chondrocytes by mediating PI3K/AKT/mTOR signaling.Bioengineered. 2021 Dec;12(1):2984-2999. doi: 10.1080/21655979.2021.1943602. Bioengineered. 2021. PMID: 34167449 Free PMC article.
References
-
- Bellomo R., Kellum J. A. & Ronco C. Acute kidney injury. Lancet 380, 756–766 (2012). - PubMed
-
- Thadhani R., Pascual M. & Bonventre J. V. Acute renal failure. New Engl J Med 334, 1448–1460 (1996). - PubMed
-
- Anathhanam S. & Lewington A. J. Acute kidney injury. J Roy Coll Phys Lond 43, 323–329 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

