Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions--a pilot study
- PMID: 26830812
- PMCID: PMC4736260
- DOI: 10.1186/s13287-016-0281-8
Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions--a pilot study
Abstract
Background: Adipose tissue-derived mesenchymal stromal cells (AT-MSCs) are frequently used to treat equine tendinopathies. Up to now, knowledge about the fate of autologous AT-MSCs after intralesional injection into equine superficial digital flexor tendons (SDFTs) is very limited. The purpose of this study was to monitor the presence of intralesionally injected autologous AT-MSCs labelled with superparamagnetic iron oxide (SPIO) nanoparticles and green fluorescent protein (GFP) over a staggered period of 3 to 9 weeks with standing magnetic resonance imaging (MRI) and histology.
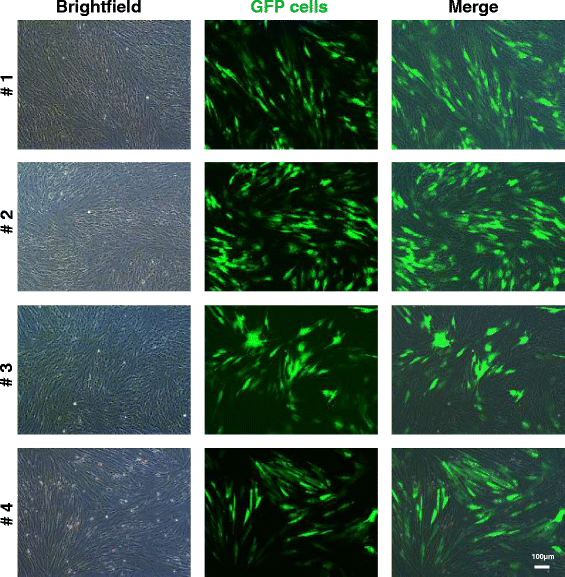
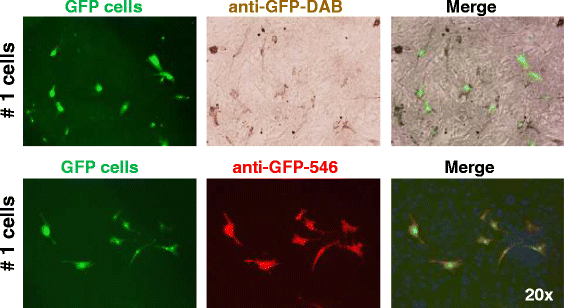
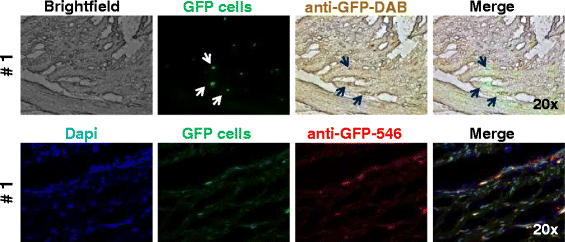
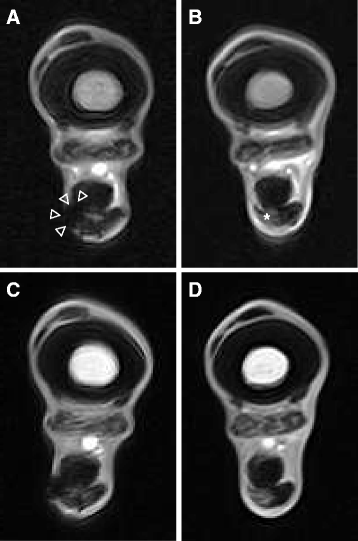
Methods: Four adult warmblood horses received a unilateral injection of 10 × 10(6) autologous AT-MSCs into surgically created front-limb SDFT lesions. Administered AT-MSCs expressed lentivirally transduced reporter genes for GFP and were co-labelled with SPIO particles in three horses. The presence of AT-MSCs in SDFTs was evaluated by repeated examinations with standing low-field MRI in two horses and post-mortem in all horses with Prussian blue staining, fluorescence microscopy and with immunofluorescence and immunohistochemistry using anti-GFP antibodies at 3, 5, 7 and 9 weeks after treatment.
Results: AT-MSCs labelled with SPIO particles were detectable in treated SDFTs during each MRI in T2*- and T1-weighted sequences until the end of the observation period. Post-mortem examinations revealed that all treated tendons contained high numbers of SPIO- and GFP-labelled cells.
Conclusions: Standing low-field MRI has the potential to track SPIO-labelled AT-MSCs successfully. Histology, fluorescence microscopy, immunofluorescence and immunohistochemistry are efficient tools to detect labelled AT-MSCs after intralesional injection into surgically created equine SDFT lesions. Intralesional injection of 10 × 10(6) AT-MSCs leads to the presence of high numbers of AT-MSCs in and around surgically created tendon lesions for up to 9 weeks. Integration of injected AT-MSCs into healing tendon tissue is an essential pathway after intralesional administration. Injection techniques have to be chosen deliberately to avoid reflux of the cell substrate injected. In vivo low-field MRI may be used as a non-invasive tool to monitor homing and engraftment of AT-MSCs in horses with tendinopathy of the SDFT.
Figures



Similar articles
-
Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial.Stem Cell Res Ther. 2017 Jun 5;8(1):129. doi: 10.1186/s13287-017-0564-8. Stem Cell Res Ther. 2017. PMID: 28583184 Free PMC article. Clinical Trial.
-
Evaluation of mesenchymal stem cell migration after equine tendonitis therapy.Equine Vet J. 2014 Sep;46(5):635-8. doi: 10.1111/evj.12173. Epub 2013 Dec 17. Equine Vet J. 2014. PMID: 23998777
-
Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon.Vet J. 2017 Jun;224:76-84. doi: 10.1016/j.tvjl.2017.04.005. Epub 2017 May 2. Vet J. 2017. PMID: 28697880
-
FluidMAG iron nanoparticle-labeled mesenchymal stem cells for tracking cell homing to tumors.2009 Dec 23 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Dec 23 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641467 Free Books & Documents. Review.
-
Amine-modified silica-coated polyhedral superparamagnetic iron oxide nanoparticle–labeled rabbit bone marrow–derived mesenchymal stem cells.2009 Dec 11 [updated 2010 Jan 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Dec 11 [updated 2010 Jan 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641854 Free Books & Documents. Review.
Cited by
-
Experimental bladder regeneration using a poly-l-lactide/silk fibroin scaffold seeded with nanoparticle-labeled allogenic bone marrow stromal cells.Int J Nanomedicine. 2016 Sep 6;11:4521-4533. doi: 10.2147/IJN.S111656. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27660444 Free PMC article.
-
A Translational Porcine Model for Human Cell-Based Therapies in the Treatment of Posttraumatic Osteoarthritis After Anterior Cruciate Ligament Injury.Am J Sports Med. 2020 Oct;48(12):3002-3012. doi: 10.1177/0363546520952353. Epub 2020 Sep 14. Am J Sports Med. 2020. PMID: 32924528 Free PMC article.
-
Biological Therapies in Regenerative Sports Medicine.Sports Med. 2017 May;47(5):807-828. doi: 10.1007/s40279-016-0620-z. Sports Med. 2017. PMID: 27677916 Review.
-
In Vivo Magic Angle Magnetic Resonance Imaging for Cell Tracking in Equine Low-Field MRI.Stem Cells Int. 2019 Dec 17;2019:5670106. doi: 10.1155/2019/5670106. eCollection 2019. Stem Cells Int. 2019. PMID: 31933650 Free PMC article.
-
Nanotechnology-Assisted Cell Tracking.Nanomaterials (Basel). 2022 Apr 20;12(9):1414. doi: 10.3390/nano12091414. Nanomaterials (Basel). 2022. PMID: 35564123 Free PMC article. Review.
References
-
- Smith R, Young N, Dudhia J, Kasashima Y, Clegg P, Goodship A. Effectiveness of bone-marrow-derived mesenchymal progenitor cells for naturally occurring tendinopathy in the horse. Regen Med. 2009;4 Suppl 2:S25.
-
- Carvalho AD, Alves ALG, de Oliveira PGG, Alvarez LEC, Amorim RL, Hussni CA, et al. Use of Adipose Tissue-Derived Mesenchymal Stem Cells for Experimental Tendinitis Therapy in Equines. J Equine Vet Sci. 2011;31:26–34. doi: 10.1016/j.jevs.2010.11.014. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

