A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5 mg
- PMID: 26830816
- PMCID: PMC4755445
- DOI: 10.1093/humrep/dev341
A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5 mg
Abstract
Study question: Will the use of levonorgestrel (LNG) 1.5 mg taken at each day of coitus by women who have relatively infrequent sex be an efficacious, safe and acceptable contraceptive method?
Summary answer: Typical use of LNG 1.5 mg taken pericoitally, before or within 24 h of sexual intercourse, provides contraceptive efficacy of up to 11.0 pregnancies per 100 women-years (W-Y) in the primary evaluable population and 7.1 pregnancies per 100 W-Y in the evaluable population.
What is known already: LNG 1.5 mg is an effective emergency contraception following unprotected intercourse. Some users take it repeatedly, as their means of regular contraception.
Study design, size, duration: This was a prospective, open-label, single-arm, multicentre Phase III trial study with women who have infrequent coitus (on up to 6 days a month). Each woman had a follow-up visit at 2.5, 4.5 and 6.5 months after admission or until pregnancy occurs if sooner, or she decided to interrupt participation. The study was conducted between 10 January 2012 and 15 November 2014.
Participants/materials, setting, methods: A total of 330 healthy fertile women aged 18-45 years at risk of pregnancy who reported sexual intercourse on up to 6 days a month, were recruited from four university centres located in Bangkok, Thailand; Campinas, Brazil; Singapore and Szeged, Hungary to use LNG 1.5 mg pericoitally (24 h before or after coitus) as their primary method of contraception. The participants were instructed to take one tablet every day she had sex, without taking more than one tablet in any 24-h period, and to maintain a paper diary for recording date and time for every coital act and ingestion of the study tablet, use of other contraceptive methods and vaginal bleeding patterns. Anaemia was assessed by haemoglobin evaluation. Pregnancy tests were performed monthly and pregnancies occurring during product use were assessed by ultrasound. At the 2.5-month and final visit at 6.5 months, acceptability questions were administered.
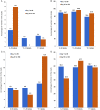
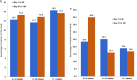
Main results and the role of chance: There were 321 women included in the evaluable population (which includes all eligible women enrolled), with 141.9 woman-years (W-Y) of observation and with a rate (95% confidence interval [CI]) of 7.1 (3.8; 13.1) pregnancies per 100 W-Y of typical use (which reflects use of the study drug as main contraceptive method, but also includes possible use of other contraceptives from admission to end of study) and 7.5 (4.0; 13.9) pregnancies per 100 W-Y of sole use. In the primary evaluable population (which includes only eligible enrolled women <35 years old), the rate was 10.3 (5.4; 19.9) pregnancies per 100 W-Y of typical use, and 11.0 (5.7; 13.1) pregnancies per 100 W-Y of sole use. There were three reported severe adverse events and 102 other mild adverse events (most common were headache, nausea, abdominal and pelvic pain), with high recovery rate. The vaginal bleeding patterns showed a slight decrease in volume of bleeding and the number of bleeding-free days increased over time. There was only one case of severe anaemia, found at the final visit (0.4%). The method was considered acceptable, as over 90% of participants would choose to use it in the future or would recommend it to others.
Limitations, reasons for caution: This was a single-arm study with small sample size, without a control group, designed as a proof of concept study to explore the feasibility of this type of contraception.
Wider implications of the findings: A larger clinical study evaluating pericoital contraception with LNG is feasible and our data show that this method would be acceptable to many women.
Study funding/competing interests: This study received partial financial support from the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR) and the World Health Organization. Gynuity and the Bill and Melinda Gates Foundation (BMGF) provided financial support for project monitoring. HRA Pharma donated the LNG product. N.K. was the initial project manager when she was with WHO/HRP and was employed by HRA Pharma, which distributes LNG for emergency contraception. The other authors declare no conflicts of interest.
Trial registration number: This study was registered on ANZCTR, Trial ID ACTRN12611001037998.
Trial registration date: 4 October 2011.
Date of first patient's enrolment: 10 January 2012.
Keywords: anaemia; contraception; effectiveness; oral levonorgestrel; pericoital.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls.Hum Reprod. 2015 Nov;30(11):2527-38. doi: 10.1093/humrep/dev221. Epub 2015 Sep 25. Hum Reprod. 2015. PMID: 26409014 Clinical Trial.
-
Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis.Lancet. 2010 Feb 13;375(9714):555-62. doi: 10.1016/S0140-6736(10)60101-8. Epub 2010 Jan 29. Lancet. 2010. PMID: 20116841 Clinical Trial.
-
A single-arm study to evaluate the efficacy, safety and acceptability of pericoital oral contraception with levonorgestrel.Contraception. 2014 Mar;89(3):215-21. doi: 10.1016/j.contraception.2013.11.013. Epub 2013 Nov 26. Contraception. 2014. PMID: 24388695 Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Interventions for emergency contraception.Cochrane Database Syst Rev. 2019 Jan 20;1(1):CD001324. doi: 10.1002/14651858.CD001324.pub6. Cochrane Database Syst Rev. 2019. PMID: 30661244 Free PMC article.
Cited by
-
Acceptability of an on-demand pericoital oral contraceptive pill: a systematic scoping review.Reprod Health. 2024 Jun 28;21(1):93. doi: 10.1186/s12978-024-01829-7. Reprod Health. 2024. PMID: 38943120 Free PMC article.
-
Centring women's voices in contraceptive innovation: building the case for an on-demand, pericoital pill.BMJ Sex Reprod Health. 2025 Jan 6;51(1):1-2. doi: 10.1136/bmjsrh-2024-202510. BMJ Sex Reprod Health. 2025. PMID: 39592198 Free PMC article. No abstract available.
-
State of emergency contraception in the U.S., 2018.Contracept Reprod Med. 2018 Sep 5;3:20. doi: 10.1186/s40834-018-0067-8. eCollection 2018. Contracept Reprod Med. 2018. PMID: 30202545 Free PMC article. Review.
-
A Systematic Review and Meta-analysis of the Adverse Effects of Levonorgestrel Emergency Oral Contraceptive.Clin Drug Investig. 2020 May;40(5):395-420. doi: 10.1007/s40261-020-00901-x. Clin Drug Investig. 2020. PMID: 32162237
-
Feasibility of e-commerce pharmacy provision and acceptability of levonorgestrel 1.5 mg for pericoital use in urban and peri-urban settings in Kenya: a prospective cohort study.BMJ Sex Reprod Health. 2023 Jan;49(1):35-42. doi: 10.1136/bmjsrh-2022-201653. Epub 2022 Nov 2. BMJ Sex Reprod Health. 2023. PMID: 36323492 Free PMC article.
References
-
- American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol 2012;120:1250–1253. - PubMed
-
- Arowojolu AO, Adekunle AO. Perception and practice of emergency contraception by post-secondary school students in southwest Nigeria. Afr J Reprod Health 2000;4:56–65. - PubMed
-
- Belsey EM, Machin D, d'Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception 1986;34:253–260. - PubMed
-
- Bhattacharjee SK, Romeo J, Kononova ES, Pretnar-Darovec A, Saraya L, Shi YE. Postcoital contraception with LNG during the peri-ovulatory phase of the menstrual cycle. Task Force on Post-ovulatory Methods for Fertility Regulation. Contraception 1987;36:275–286. - PubMed
-
- Croxatto HB, Devoto L, Durand M, Ezcurra E, Larrea F, Nagle C, Ortiz ME, Vantman D, Vega M, von Hertzen H. Mechanism of action of hormonal preparations used for emergency contraception: a review of the literature. Contraception 2001;63:111–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

