Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABAA Receptors by Propofol
- PMID: 26830963
- PMCID: PMC5050400
- DOI: 10.2174/1570159x14666160202121319
Multiple Non-Equivalent Interfaces Mediate Direct Activation of GABAA Receptors by Propofol
Abstract
Background: Propofol is a sedative agent that at clinical concentrations acts by allosterically activating or potentiating the γ-aminobutyric acid type A (GABAA) receptor. Mutational, modeling, and photolabeling studies with propofol and its analogues have identified potential interaction sites in the transmembrane domain of the receptor. At the "+" of the β subunit, in the β-α interface, meta-azipropofol labels the M286 residue in the third transmembrane domain. Substitution of this residue with tryptophan results in loss of potentiation by propofol. At the "-" side of the β subunit, in the α-β interface (or β-β interface, in the case of homomeric β receptors), ortho-propofol diazirine labels the H267 residue in the second transmembrane domain. Structural modeling indicates that the β(H267) residue lines a cavity that docks propofol with favorable interaction energy.
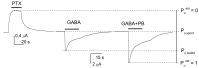
Method: We used two-electrode voltage clamp to determine the functional effects of mutations to the "+" and "-" sides of the β subunit on activation of the α1β3 GABAA receptor by propofol.
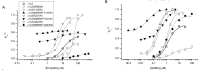
Results: We found that while the individual mutations had a small effect, the combination of the M286W mutation with tryptophan mutations of selected residues at the α-β interface leads to strong reduction in gating efficacy for propofol.
Conclusion: We conclude that α1β3 GABAA receptors can be activated by propofol interactions with the β-β, α-β, and β-α interfaces, where distinct, non-equivalent regions control channel gating. Any interface can mediate activation, hence substitutions at all interfaces are required for loss of activation by propofol.
Figures


References
-
- Belelli D., Callachan H., Hill-Venning C., Peters J.A., Lambert J.J. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br. J. Pharmacol. 1996;118(3):563–576. [http:// dx.doi.org/10.1111/j.1476-5381.1996.tb15439.x]. [PMID: 8762079]. - PMC - PubMed
-
- Harrison N.L., Simmonds M.A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323(2):287–292. [http://dx.doi.org/10.1016/0006-8993(84)90299-3]. [PMID: 6098342]. - PubMed
-
- Cestari I.N., Uchida I., Li L., Burt D., Yang J. The agonistic action of pentobarbital on GABAA β-subunit homomeric receptors. Neuroreport. 1996;7(4):943–947. [http://dx.doi.org/10.1097/ 00001756-199603220-00023]. [PMID: 8724679]. - PubMed
-
- Krishek B.J., Moss S.J., Smart T.G. Homomeric β 1 γ-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol. Pharmacol. 1996;49(3):494–504. [PMID: 8643089]. - PubMed
-
- Amin J., Weiss D.S. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366(6455):565–569. [http://dx.doi.org/ 10.1038/366565a0]. [PMID: 7504783]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
