Distinct Reward Properties are Encoded via Corticostriatal Interactions
- PMID: 26831208
- PMCID: PMC4735713
- DOI: 10.1038/srep20093
Distinct Reward Properties are Encoded via Corticostriatal Interactions
Abstract
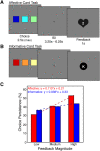
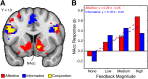
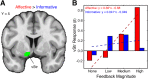
The striatum serves as a critical brain region for reward processing. Yet, understanding the link between striatum and reward presents a challenge because rewards are composed of multiple properties. Notably, affective properties modulate emotion while informative properties help obtain future rewards. We approached this problem by emphasizing affective and informative reward properties within two independent guessing games. We found that both reward properties evoked activation within the nucleus accumbens, a subregion of the striatum. Striatal responses to informative, but not affective, reward properties predicted subsequent utilization of information for obtaining monetary reward. We hypothesized that activation of the striatum may be necessary but not sufficient to encode distinct reward properties. To investigate this possibility, we examined whether affective and informative reward properties were differentially encoded in corticostriatal interactions. Strikingly, we found that the striatum exhibited dissociable connectivity patterns with the ventrolateral prefrontal cortex, with increasing connectivity for affective reward properties and decreasing connectivity for informative reward properties. Our results demonstrate that affective and informative reward properties are encoded via corticostriatal interactions. These findings highlight how corticostriatal systems contribute to reward processing, potentially advancing models linking striatal activation to behavior.
Figures

References
-
- Smith D. V. & Delgado M. R. In Brain Mapping: An Encyclopedic Reference Vol. 3 (ed. Toga A. W. ) 361–366 (Academic Press, 2015).
-
- O’Doherty J., Kringelbach M. L., Rolls E. T., Hornak J. & Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4, 95–102 (2001). - PubMed
-
- Delgado M. R., Nystrom L. E., Fissell C., Noll D. C. & Fiez J. A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 84, 3072–3077 (2000). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

