The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters
- PMID: 26831515
- PMCID: PMC4814305
- DOI: 10.1042/BJ20151120
The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters
Abstract
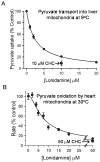
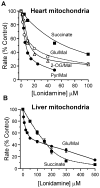
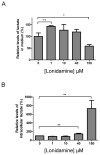
Lonidamine (LND) is an anti-tumour drug particularly effective at selectively sensitizing tumours to chemotherapy, hyperthermia and radiotherapy, although its precise mode of action remains unclear. It has been reported to perturb the bioenergetics of cells by inhibiting glycolysis and mitochondrial respiration, whereas indirect evidence suggests it may also inhibit L-lactic acid efflux from cells mediated by members of the proton-linked monocarboxylate transporter (MCT) family and also pyruvate uptake into the mitochondria by the mitochondrial pyruvate carrier (MPC). In the present study, we test these possibilities directly. We demonstrate that LND potently inhibits MPC activity in isolated rat liver mitochondria (Ki2.5 μM) and co-operatively inhibits L-lactate transport by MCT1, MCT2 and MCT4 expressed in Xenopus laevisoocytes with K0.5 and Hill coefficient values of 36-40 μM and 1.65-1.85 respectively. In rat heart mitochondria LND inhibited the MPC with similar potency and uncoupled oxidation of pyruvate was inhibited more effectively (IC50~ 7 μM) than other substrates including glutamate (IC50~ 20 μM). In isolated DB-1 melanoma cells 1-10 μM LND increased L-lactate output, consistent with MPC inhibition, but higher concentrations (150 μM) decreased L-lactate output whereas increasing intracellular [L-lactate] > 5-fold, consistent with MCT inhibition. We conclude that MPC inhibition is the most sensitive anti-tumour target for LND, with additional inhibitory effects on MCT-mediated L-lactic acid efflux and glutamine/glutamate oxidation. Together these actions can account for published data on the selective tumour effects of LND onL-lactate, intracellular pH (pHi) and ATP levels that can be partially mimicked by the established MPC and MCT inhibitor α-cyano-4-hydroxycinnamate (CHC).
Keywords: bioenergetics; cancer; metabolism; mitochondrial pyruvate carrier; monocarboxylate transporter; tumour acidification.
© 2016 Authors; published by Portland Press Limited.
Conflict of interest statement
None
Figures

References
-
- Cioli V, Bellocci B, Putzolu S, Malorni W, Demartino C. Anti-spermogenic activity of Lonidamine (AF-1890) in rabbit. Ultramicroscopy. 1980;5:418–418.
-
- Band PR, Maroun J, Pritchard K, Stewart D, Coppin CM, Wilson K, Eisenhauer EA. Phase II study of lonidamine in patients with metastatic breast cancer: a National Cancer Institute of Canada Clinical Trials Group Study. Cancer Treat Rep. 1986;70:1305–1310. - PubMed
-
- Weinerman BH, Eisenhauer EA, Besner JG, Coppin CM, Stewart D, Band PR. Phase II study of lonidamine in patients with metastatic renal cell carcinoma: a National Cancer Institute of Canada Clinical Trials Group Study. Cancer Treat Rep. 1986;70:751–754. - PubMed
-
- Nath K, Nelson DS, Ho AM, Lee SC, Darpolor MM, Pickup S, Zhou R, Heitjan DF, Leeper DB, Glickson JD. (31) P and (1) H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26:98–105. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

