Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas
- PMID: 26832770
- PMCID: PMC4909128
- DOI: 10.1093/jnci/djv427
Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas
Abstract
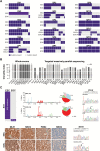
Synchronous early-stage endometrioid endometrial carcinomas (EECs) and endometrioid ovarian carcinomas (EOCs) are associated with a favorable prognosis and have been suggested to represent independent primary tumors rather than metastatic disease. We subjected sporadic synchronous EECs/EOCs from five patients to whole-exome massively parallel sequencing, which revealed that the EEC and EOC of each case displayed strikingly similar repertoires of somatic mutations and gene copy number alterations. Despite the presence of mutations restricted to the EEC or EOC in each case, we observed that the mutational processes that shaped their respective genomes were consistent. High-depth targeted massively parallel sequencing of sporadic synchronous EECs/EOCs from 17 additional patients confirmed that these lesions are clonally related. In an additional Lynch Syndrome case, however, the EEC and EOC were found to constitute independent cancers lacking somatic mutations in common. Taken together, sporadic synchronous EECs/EOCs are clonally related and likely constitute dissemination from one site to the other.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Making a Difference: Distinguishing Two Primaries From Metastasis in Synchronous Tumors of the Ovary and Uterus.J Natl Cancer Inst. 2016 Feb 1;108(6):djv442. doi: 10.1093/jnci/djv442. Print 2016 Jun. J Natl Cancer Inst. 2016. PMID: 26832772
References
-
- Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83(2):355–362. - PubMed
-
- Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC; 2014.
-
- Soliman PT, Slomovitz BM, Broaddus RR, et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol. 2004;94(2):456–462. - PubMed
-
- Ramus SJ, Elmasry K, Luo Z, et al. Predicting clinical outcome in patients diagnosed with synchronous ovarian and endometrial cancer. Clin Cancer Res. 2008;14(18):5840–5848. - PubMed
-
- Ulbright TM, Roth LM. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Hum Pathol. 1985;16(1):28–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

