Perspectives on the design and methodology of periconceptional nutrient supplementation trials
- PMID: 26833080
- PMCID: PMC4736099
- DOI: 10.1186/s13063-015-1124-0
Perspectives on the design and methodology of periconceptional nutrient supplementation trials
Abstract
Periconceptional supplementation could extend the period over which maternal and fetal nutrition is improved, but there are many challenges facing early-life intervention studies. Periconceptional trials differ from pregnancy supplementation trials, not only because of the very early or pre-gestational timing of nutrient exposure but also because they generate subsidiary information on participants who remain non-pregnant. The methodological challenges are more complex although, if well designed, they provide opportunities to evaluate concurrent hypotheses related to the health of non-pregnant women, especially nulliparous adolescents. This review examines the framework of published and ongoing randomised trial designs. Four cohorts typically arise from the periconceptional trial design--two of which are non-pregnant and two are pregnant--and this structure provides assessment options related to pre-pregnant, maternal, pregnancy and fetal outcomes. Conceptually the initial decision for single or micronutrient intervention is central--as is the choice of dosage and content--in order to establish a comparative framework across trials, improve standardisation, and facilitate interpretation of mechanistic hypotheses. Other trial features considered in the review include: measurement options for baseline and outcome assessments; adherence to long-term supplementation; sample size considerations in relation to duration of nutrient supplementation; cohort size for non-pregnant and pregnant cohorts as the latter is influenced by parity selection; integrating qualitative studies and data management issues. Emphasis is given to low resource settings where high infection rates and the possibility of nutrient-infection interactions may require appropriate safety monitoring. The focus is on pragmatic issues that may help investigators planning a periconceptional trial.
Figures
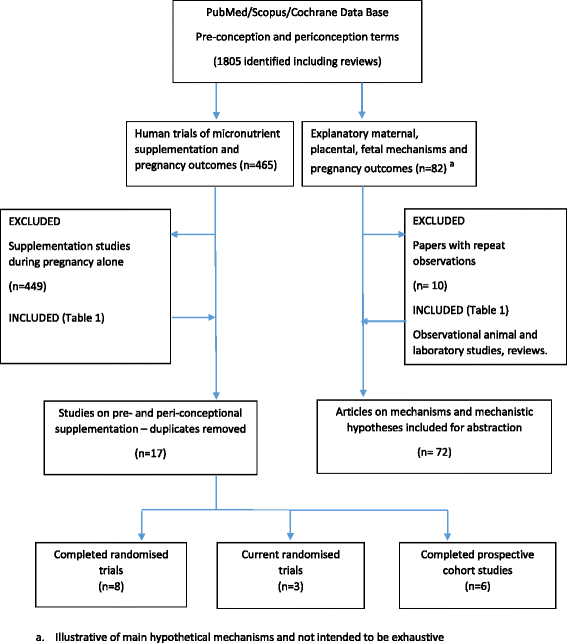


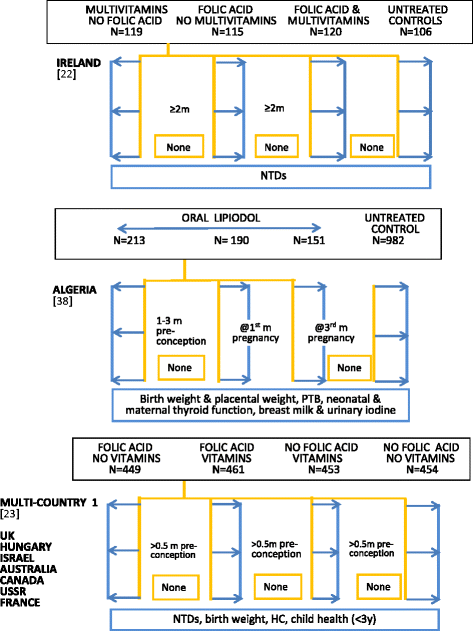
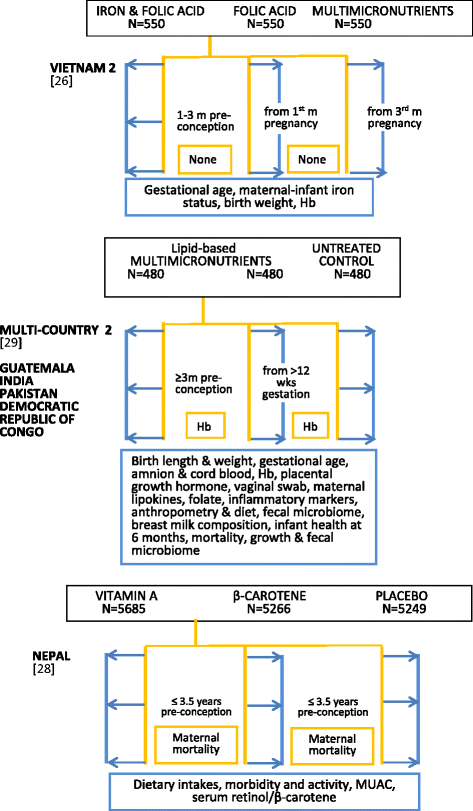
References
-
- Ronsmans C, Fisher DJ, Osmond C, Margetts BM. Fall CHD for the Maternal Micronutrient Supplementation Group (MMSSG). Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr Bull. 2009;30:S547–26. doi: 10.1177/15648265090304S409. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

