Bidirectional Transfer of Raltegravir in an Ex Vivo Human Cotyledon Perfusion Model
- PMID: 26833154
- PMCID: PMC4862479
- DOI: 10.1128/AAC.00007-16
Bidirectional Transfer of Raltegravir in an Ex Vivo Human Cotyledon Perfusion Model
Abstract
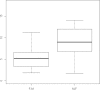
Placental transfer of the HIV integrase inhibitor raltegravir (RLT) was investigated in term human cotyledons in the maternal-to-fetal (n = 3) and fetal-to-maternal (n = 6) directions. In the maternal-to-fetal direction, the mean ± standard deviation (SD) fetal transfer rate (FTR) was 9.1% ± 1.4%, and the mean ± SD clearance index (IC), i.e., RLT FTR/antipyrine FTR, was 0.28 ± 0.05. In the fetal-to-maternal direction, the mean ± SD CI was 0.31 ± 0.09. Placental transfer of RLT was high in both directions.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Mandelbrot L, Tubiana R, LeChenadec J, Dollfus C, Faye A, Pannier E, Matheron S, Khuong MA, Garrait V, Reliquet V, Devidas A, Berrebi A, Allisy C, Elleau C, Arvieux C, Rouzioux C, Warszawski J, Blanche S, ANRS-EPF Study Group. 2015. No perinatal transmission of HIV-1 from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 61:1715–1725. doi: 10.1093/cid/civ578. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

