Cell Wall Changes in Amphotericin B-Resistant Strains from Candida tropicalis and Relationship with the Immune Responses Elicited by the Host
- PMID: 26833156
- PMCID: PMC4808153
- DOI: 10.1128/AAC.02681-15
Cell Wall Changes in Amphotericin B-Resistant Strains from Candida tropicalis and Relationship with the Immune Responses Elicited by the Host
Abstract
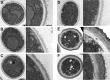
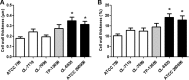
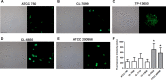
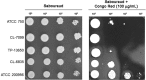
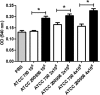
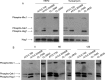
We have morphologically characterizedCandida tropicalisisolates resistant to amphotericin B (AmB). These isolates present an enlarged cell wall compared to isolates of regular susceptibility. This correlated with higher levels of β-1,3-glucan in the cell wall but not with detectable changes in chitin content. In line with this, AmB-resistant strains showed reduced susceptibility to Congo red. Moreover, mitogen-activated protein kinases (MAPKs) involved in cell integrity were already activated during regular growth in these strains. Finally, we investigated the response elicited by human blood cells and found that AmB-resistant strains induced a stronger proinflammatory response than susceptible strains. In agreement, AmB-resistant strains also induced stronger melanization ofGalleria mellonellalarvae, indicating that the effect of alterations of the cell wall on the immune response is conserved in different types of hosts. Our results suggest that resistance to AmB is associated with pleiotropic mechanisms that might have important consequences, not only for the efficacy of the treatment but also for the immune response elicited by the host.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Relation between cell wall chitin content and susceptibility to amphotericin B in Kluyveromyces, Candida and Schizosaccharomyces species.Res Microbiol. 2003 Apr;154(3):215-22. doi: 10.1016/S0923-2508(03)00049-4. Res Microbiol. 2003. PMID: 12706511
-
Tolerance to amphotericin B in clinical isolates of Candida tropicalis.Diagn Microbiol Infect Dis. 2004 Nov;50(3):179-85. doi: 10.1016/j.diagmicrobio.2004.06.002. Diagn Microbiol Infect Dis. 2004. PMID: 15541603
-
Blocking Hsp70 enhances the efficiency of amphotericin B treatment against resistant Aspergillus terreus strains.Antimicrob Agents Chemother. 2015 Jul;59(7):3778-88. doi: 10.1128/AAC.05164-14. Epub 2015 Apr 13. Antimicrob Agents Chemother. 2015. PMID: 25870060 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Candida tropicalis in human disease.Crit Rev Microbiol. 2010 Nov;36(4):282-98. doi: 10.3109/1040841X.2010.489506. Crit Rev Microbiol. 2010. PMID: 20883082 Review.
Cited by
-
Antifungal Activity of Chitosan against Histoplasma capsulatum in Planktonic and Biofilm Forms: A Therapeutic Strategy in the Future?J Fungi (Basel). 2023 Dec 15;9(12):1201. doi: 10.3390/jof9121201. J Fungi (Basel). 2023. PMID: 38132801 Free PMC article.
-
Fungal Cell Wall: Emerging Antifungals and Drug Resistance.Front Microbiol. 2019 Nov 21;10:2573. doi: 10.3389/fmicb.2019.02573. eCollection 2019. Front Microbiol. 2019. PMID: 31824443 Free PMC article. Review.
-
Epigenetic Regulation of Antifungal Drug Resistance.J Fungi (Basel). 2022 Aug 19;8(8):875. doi: 10.3390/jof8080875. J Fungi (Basel). 2022. PMID: 36012862 Free PMC article. Review.
-
Iron Assimilation during Emerging Infections Caused by Opportunistic Fungi with emphasis on Mucorales and the Development of Antifungal Resistance.Genes (Basel). 2020 Oct 30;11(11):1296. doi: 10.3390/genes11111296. Genes (Basel). 2020. PMID: 33143139 Free PMC article. Review.
-
Cell Wall-Associated Virulence Factors Contribute to Increased Resilience of Old Cryptococcus neoformans Cells.Front Microbiol. 2019 Nov 7;10:2513. doi: 10.3389/fmicb.2019.02513. eCollection 2019. Front Microbiol. 2019. PMID: 31787940 Free PMC article.
References
-
- Finkelstein A, Holz R. 1973. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes 2:377–408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

