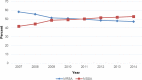
Linezolid Surveillance Results for the United States (LEADER Surveillance Program 2014)
- PMID: 26833165
- PMCID: PMC4808230
- DOI: 10.1128/AAC.02803-15
Linezolid Surveillance Results for the United States (LEADER Surveillance Program 2014)
Abstract
Thelinezolidexperience andaccuratedetermination ofresistance (LEADER) surveillance program has monitored linezolid activity, spectrum, and resistance since 2004. In 2014, a total of 6,865 Gram-positive pathogens from 60 medical centers from 36 states were submitted. The organism groups evaluated wereStaphylococcus aureus(3,106), coagulase-negative staphylococci (CoNS; 797), enterococci (855),Streptococcus pneumoniae(874), viridans group streptococci (359), and beta-hemolytic streptococci (874). Susceptibility testing was performed by reference broth microdilution at the monitoring laboratory. Linezolid-resistant isolates were confirmed by repeat testing. PCR and sequencing were performed to detect mutations in 23S rRNA, L3, L4, and L22 proteins and acquired genes (cfrandoptrA). The MIC50/90forStaphylococcus aureuswas 1/1 μg/ml, with 47.2% of isolates being methicillin-resistantStaphylococcus aureus Linezolid was active against allStreptococcus pneumoniaestrains and beta-hemolytic streptococci with a MIC50/90of 1/1 μg/ml and against viridans group streptococci with a MIC50/90of 0.5/1 μg/ml. Among the linezolid-nonsusceptible MRSA strains, one strain harboredcfronly (MIC, 4 μg/ml), one harbored G2576T (MIC, 8 μg/ml), and one containedcfrand G2576T with L3 changes (MIC, ≥8 μg/ml). Among CoNS, 0.75% (six isolates) of all strains demonstrated linezolid MIC results of ≥4 μg/ml. Five of these were identified asStaphylococcus epidermidis, four of which containedcfrin addition to the presence of mutations in the ribosomal proteins L3 and L4, alone or in combination with 23S rRNA (G2576T) mutations. Six enterococci (0.7%) were linezolid nonsusceptible (≥4 μg/ml; five with G2576T mutations, including one with an additionalcfrgene, and one strain withoptrAonly). Linezolid demonstrated excellent activity and a sustained susceptibility rate of 99.78% overall.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States.Antimicrob Agents Chemother. 2011 Aug;55(8):3684-90. doi: 10.1128/AAC.01729-10. Epub 2011 Jun 13. Antimicrob Agents Chemother. 2011. PMID: 21670176 Free PMC article.
-
LEADER surveillance program results for 2010: an activity and spectrum analysis of linezolid using 6801 clinical isolates from the United States (61 medical centers).Diagn Microbiol Infect Dis. 2012 Sep;74(1):54-61. doi: 10.1016/j.diagmicrobio.2012.05.012. Epub 2012 Jun 15. Diagn Microbiol Infect Dis. 2012. PMID: 22704791
-
Zyvox Annual Appraisal of Potency and Spectrum program: linezolid surveillance program results for 2008.Diagn Microbiol Infect Dis. 2009 Dec;65(4):404-13. doi: 10.1016/j.diagmicrobio.2009.10.001. Diagn Microbiol Infect Dis. 2009. PMID: 19913683
-
Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms.Drug Resist Updat. 2014 Apr;17(1-2):1-12. doi: 10.1016/j.drup.2014.04.002. Epub 2014 Apr 6. Drug Resist Updat. 2014. PMID: 24880801 Review.
-
In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010).Clin Infect Dis. 2012 Sep;55 Suppl 3:S206-14. doi: 10.1093/cid/cis563. Clin Infect Dis. 2012. PMID: 22903953 Review.
Cited by
-
Effect of ZnO nanoparticles on methicillin, vancomycin, linezolid resistance and biofilm formation in Staphylococcus aureus isolates.Ann Clin Microbiol Antimicrob. 2021 Aug 21;20(1):54. doi: 10.1186/s12941-021-00459-2. Ann Clin Microbiol Antimicrob. 2021. PMID: 34419054 Free PMC article.
-
Five-Year Summary of In Vitro Activity and Resistance Mechanisms of Linezolid against Clinically Important Gram-Positive Cocci in the United States from the LEADER Surveillance Program (2011 to 2015).Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00609-17. doi: 10.1128/AAC.00609-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28483950 Free PMC article.
-
Newer antibiotics for the treatment of peritoneal dialysis-related peritonitis.Clin Kidney J. 2016 Aug;9(4):616-23. doi: 10.1093/ckj/sfw059. Epub 2016 Jul 4. Clin Kidney J. 2016. PMID: 27478608 Free PMC article.
-
Faecal carriage of enterococci harbouring oxazolidinone resistance genes among healthy humans in the community in Switzerland.J Antimicrob Chemother. 2022 Sep 30;77(10):2779-2783. doi: 10.1093/jac/dkac260. J Antimicrob Chemother. 2022. PMID: 35971252 Free PMC article.
-
Nationwide Surveillance of Novel Oxazolidinone Resistance Gene optrA in Enterococcus Isolates in China from 2004 to 2014.Antimicrob Agents Chemother. 2016 Nov 21;60(12):7490-7493. doi: 10.1128/AAC.01256-16. Print 2016 Dec. Antimicrob Agents Chemother. 2016. PMID: 27645239 Free PMC article.
References
-
- Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from the United States (56 medical centers). Antimicrob Agents Chemother 55:3684–3690. doi:10.1128/AAC.01729-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical