A clickable psoralen to directly quantify DNA interstrand crosslinking and repair
- PMID: 26833244
- PMCID: PMC4762217
- DOI: 10.1016/j.bmc.2016.01.032
A clickable psoralen to directly quantify DNA interstrand crosslinking and repair
Abstract
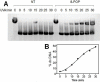
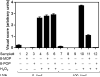
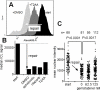
DNA interstrand crosslinks (ICLs) represent physical obstacles to advancing replication forks and transcription complexes. A range of ICL-inducing agents have successfully been incorporated into cancer therapeutics. While studies have adopted UVA-activated psoralens as model ICL-inducing agents for investigating ICL repair, direct detection of the lesion has often been tempered by tagging the psoralen scaffold with a relatively large reporter group that may perturb the biological activity of the parent psoralen. Here a minimally-modified psoralen probe was prepared featuring a small alkyne handle suitable for click chemistry. The psoralen probe, designated 8-propargyloxypsoralen (8-POP), can be activated by UVA in vitro to generate ICLs that are susceptible to post-labeling with an azide-tagged fluorescent reporter via a copper-catalyzed reaction. A modified alkaline comet assay demonstrated that UVA-activated 8-POP proficiently generated ICLs in cells. Cellular 8-POP-DNA lesions were amenable to click-mediated ligation to fluorescent reporters in situ, which permitted their detection and quantitation by fluorescence microscopy and flow cytometry. Small molecule DNA repair inhibitors to 8-POP-treated cells attenuated the removal of 8-POP-DNA lesions, validating 8-POP as an appropriate probe for investigating cellular ICL repair. The post-labeling strategy applied in this study is inexpensive, rapid and highly modular in nature with the potential for multiple applications in DNA repair studies.
Keywords: Chemical screening; Click chemistry; DNA repair; Imaging; Interstrand DNA crosslink; Psoralen.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
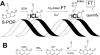


Similar articles
-
NEIL1 responds and binds to psoralen-induced DNA interstrand crosslinks.J Biol Chem. 2013 May 3;288(18):12426-36. doi: 10.1074/jbc.M113.456087. Epub 2013 Mar 18. J Biol Chem. 2013. PMID: 23508956 Free PMC article.
-
Quantification and repair of psoralen-induced interstrand crosslinks in human cells.Toxicol Lett. 2014 May 2;226(3):343-50. doi: 10.1016/j.toxlet.2014.01.019. Epub 2014 Feb 5. Toxicol Lett. 2014. PMID: 24508309
-
Pyrene chromophores for the photoreversal of psoralen interstrand crosslinks.Org Biomol Chem. 2014 Jul 28;12(28):5260-6. doi: 10.1039/c4ob00603h. Org Biomol Chem. 2014. PMID: 24922335
-
Current approaches for RNA labeling in vitro and in cells based on click reactions.Chembiochem. 2014 Nov 3;15(16):2342-7. doi: 10.1002/cbic.201402240. Epub 2014 Sep 15. Chembiochem. 2014. PMID: 25224574 Review.
-
Click chemistry-based functionalization on non-oxidized silicon substrates.Chem Asian J. 2011 Oct 4;6(10):2592-605. doi: 10.1002/asia.201100294. Epub 2011 Jul 12. Chem Asian J. 2011. PMID: 21751406 Review.
Cited by
-
A clickable melphalan for monitoring DNA interstrand crosslink accumulation and detecting ICL repair defects in Fanconi anemia patient cells.Nucleic Acids Res. 2023 Aug 25;51(15):7988-8004. doi: 10.1093/nar/gkad559. Nucleic Acids Res. 2023. PMID: 37395445 Free PMC article.
-
Repair of genomic interstrand crosslinks.DNA Repair (Amst). 2024 Sep;141:103739. doi: 10.1016/j.dnarep.2024.103739. Epub 2024 Jul 30. DNA Repair (Amst). 2024. PMID: 39106540 Free PMC article. Review.
-
Synthesis of DNA Duplexes Containing Site-Specific Interstrand Cross-Links via Sequential Reductive Amination Reactions Involving Diamine Linkers and Abasic Sites on Complementary Oligodeoxynucleotides.Chem Res Toxicol. 2021 Nov 15;34(11):2384-2391. doi: 10.1021/acs.chemrestox.1c00293. Epub 2021 Oct 25. Chem Res Toxicol. 2021. PMID: 34694787 Free PMC article.
-
Chemical Analysis of DNA Damage.Anal Chem. 2018 Jan 2;90(1):556-576. doi: 10.1021/acs.analchem.7b04247. Epub 2017 Nov 7. Anal Chem. 2018. PMID: 29084424 Free PMC article. Review. No abstract available.
-
Physicochemical Investigation of Psoralen Binding to Double Stranded DNA through Electroanalytical and Cheminformatic Approaches.Pharmaceuticals (Basel). 2020 May 28;13(6):108. doi: 10.3390/ph13060108. Pharmaceuticals (Basel). 2020. PMID: 32481669 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

