De Novo Loss-of-Function Mutations in USP9X Cause a Female-Specific Recognizable Syndrome with Developmental Delay and Congenital Malformations
- PMID: 26833328
- PMCID: PMC4746365
- DOI: 10.1016/j.ajhg.2015.12.015
De Novo Loss-of-Function Mutations in USP9X Cause a Female-Specific Recognizable Syndrome with Developmental Delay and Congenital Malformations
Abstract
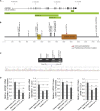
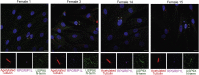
Mutations in more than a hundred genes have been reported to cause X-linked recessive intellectual disability (ID) mainly in males. In contrast, the number of identified X-linked genes in which de novo mutations specifically cause ID in females is limited. Here, we report 17 females with de novo loss-of-function mutations in USP9X, encoding a highly conserved deubiquitinating enzyme. The females in our study have a specific phenotype that includes ID/developmental delay (DD), characteristic facial features, short stature, and distinct congenital malformations comprising choanal atresia, anal abnormalities, post-axial polydactyly, heart defects, hypomastia, cleft palate/bifid uvula, progressive scoliosis, and structural brain abnormalities. Four females from our cohort were identified by targeted genetic testing because their phenotype was suggestive for USP9X mutations. In several females, pigment changes along Blaschko lines and body asymmetry were observed, which is probably related to differential (escape from) X-inactivation between tissues. Expression studies on both mRNA and protein level in affected-female-derived fibroblasts showed significant reduction of USP9X level, confirming the loss-of-function effect of the identified mutations. Given that some features of affected females are also reported in known ciliopathy syndromes, we examined the role of USP9X in the primary cilium and found that endogenous USP9X localizes along the length of the ciliary axoneme, indicating that its loss of function could indeed disrupt cilium-regulated processes. Absence of dysregulated ciliary parameters in affected female-derived fibroblasts, however, points toward spatiotemporal specificity of ciliary USP9X (dys-)function.
Copyright © 2016 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

