Assessment of the Safety and Immunogenicity of 2 Novel Vaccine Platforms for HIV-1 Prevention: A Randomized Trial
- PMID: 26833336
- PMCID: PMC5034222
- DOI: 10.7326/M15-0880
Assessment of the Safety and Immunogenicity of 2 Novel Vaccine Platforms for HIV-1 Prevention: A Randomized Trial
Abstract
Background: A prophylactic HIV-1 vaccine is a global health priority.
Objective: To assess a novel vaccine platform as a prophylactic HIV-1 regimen.
Design: Randomized, double-blind, placebo-controlled trial. Both participants and study personnel were blinded to treatment allocation. (ClinicalTrials.gov: NCT01215149).
Setting: United States, East Africa, and South Africa.
Patients: Healthy adults without HIV infection.
Intervention: 2 HIV-1 vaccines (adenovirus serotype 26 with an HIV-1 envelope A insert [Ad26.EnvA] and adenovirus serotype 35 with an HIV-1 envelope A insert [Ad35.Env], both administered at a dose of 5 × 1010 viral particles) in homologous and heterologous combinations.
Measurements: Safety and immunogenicity and the effect of baseline vector immunity.
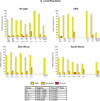
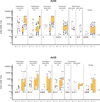
Results: 217 participants received at least 1 vaccination, and 210 (>96%) completed follow-up. No vaccine-associated serious adverse events occurred. All regimens were generally well-tolerated. All regimens elicited humoral and cellular immune responses in nearly all participants. Preexisting Ad26- or Ad35-neutralizing antibody titers had no effect on vaccine safety and little effect on immunogenicity. In both homologous and heterologous regimens, the second vaccination significantly increased EnvA antibody titers (approximately 20-fold from the median enzyme-linked immunosorbent assay titers of 30-300 to 3000). The heterologous regimen of Ad26-Ad35 elicited significantly higher EnvA antibody titers than Ad35-Ad26. T-cell responses were modest and lower in East Africa than in South Africa and the United States.
Limitations: Because the 2 envelope inserts were not identical, the boosting responses were complex to interpret. Durability of the immune responses elicited beyond 1 year is unknown.
Conclusion: Both vaccines elicited significant immune responses in all populations. Baseline vector immunity did not significantly affect responses. Second vaccinations in all regimens significantly boosted EnvA antibody titers, although vaccine order in the heterologous regimen had a modest effect on the immune response.
Primary funding source: International AIDS Vaccine Initiative, National Institutes of Health, Ragon Institute, Crucell Holland.
Figures

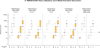
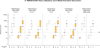

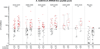
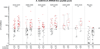
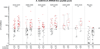
Similar articles
-
Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19).Lancet. 2018 Jul 21;392(10143):232-243. doi: 10.1016/S0140-6736(18)31364-3. Epub 2018 Jul 6. Lancet. 2018. PMID: 30047376 Free PMC article. Clinical Trial.
-
Comparison of shortened mosaic HIV-1 vaccine schedules: a randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) and a preclinical study in rhesus monkeys (NHP 17-22).Lancet HIV. 2020 Jun;7(6):e410-e421. doi: 10.1016/S2352-3018(20)30001-1. Epub 2020 Feb 17. Lancet HIV. 2020. PMID: 32078815 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of a rAd35-EnvA Prototype HIV-1 Vaccine in Combination with rAd5-EnvA in Healthy Adults (VRC 012).PLoS One. 2016 Nov 15;11(11):e0166393. doi: 10.1371/journal.pone.0166393. eCollection 2016. PLoS One. 2016. PMID: 27846256 Free PMC article. Clinical Trial.
-
Novel prime-boost vaccine strategies against HIV-1.Expert Rev Vaccines. 2019 Aug;18(8):765-779. doi: 10.1080/14760584.2019.1640117. Epub 2019 Jul 9. Expert Rev Vaccines. 2019. PMID: 31271322 Review.
-
HIV vaccines: progress to date.Drugs. 2011 Mar 5;71(4):387-414. doi: 10.2165/11585400-000000000-00000. Drugs. 2011. PMID: 21395355 Review.
Cited by
-
A multigene typing system for human adenoviruses reveals a new genotype in a collection of Swedish clinical isolates.PLoS One. 2018 Dec 14;13(12):e0209038. doi: 10.1371/journal.pone.0209038. eCollection 2018. PLoS One. 2018. PMID: 30550551 Free PMC article.
-
Integrated single-cell analysis revealed immune dynamics during Ad5-nCoV immunization.Cell Discov. 2021 Aug 10;7(1):64. doi: 10.1038/s41421-021-00300-2. Cell Discov. 2021. PMID: 34373443 Free PMC article.
-
Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.preF-Based Vaccine Combinations: A Randomized, Double-blind, Placebo-Controlled, Phase 1/2a Study.J Infect Dis. 2024 Jan 12;229(1):19-29. doi: 10.1093/infdis/jiad220. J Infect Dis. 2024. PMID: 37433021 Free PMC article. Clinical Trial.
-
Role of homologous recombination/recombineering on human adenovirus genome engineering: Not the only but the most competent solution.Eng Microbiol. 2024 Feb 8;4(1):100140. doi: 10.1016/j.engmic.2024.100140. eCollection 2024 Mar. Eng Microbiol. 2024. PMID: 39628785 Free PMC article. Review.
-
Novel viral vectors in infectious diseases.Immunology. 2018 Jan;153(1):1-9. doi: 10.1111/imm.12829. Epub 2017 Sep 26. Immunology. 2018. PMID: 28869761 Free PMC article. Review.
References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
