Modeling immune functions of the mouse blood-cerebrospinal fluid barrier in vitro: primary rather than immortalized mouse choroid plexus epithelial cells are suited to study immune cell migration across this brain barrier
- PMID: 26833402
- PMCID: PMC4734852
- DOI: 10.1186/s12987-016-0027-0
Modeling immune functions of the mouse blood-cerebrospinal fluid barrier in vitro: primary rather than immortalized mouse choroid plexus epithelial cells are suited to study immune cell migration across this brain barrier
Abstract
Background: The blood-cerebrospinal fluid barrier (BCSFB) established by the choroid plexus (CP) epithelium has been recognized as a potential entry site of immune cells into the central nervous system during immunosurveillance and neuroinflammation. The location of the choroid plexus impedes in vivo analysis of immune cell trafficking across the BCSFB. Thus, research on cellular and molecular mechanisms of immune cell migration across the BCSFB is largely limited to in vitro models. In addition to forming contact-inhibited epithelial monolayers that express adhesion molecules, the optimal in vitro model must establish a tight permeability barrier as this influences immune cell diapedesis.
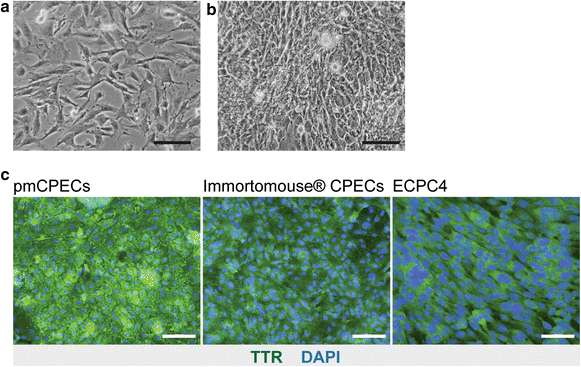
Methods: We compared cell line models of the mouse BCSFB derived from the Immortomouse(®) and the ECPC4 line to primary mouse choroid plexus epithelial cell (pmCPEC) cultures for their ability to establish differentiated and tight in vitro models of the BCSFB.
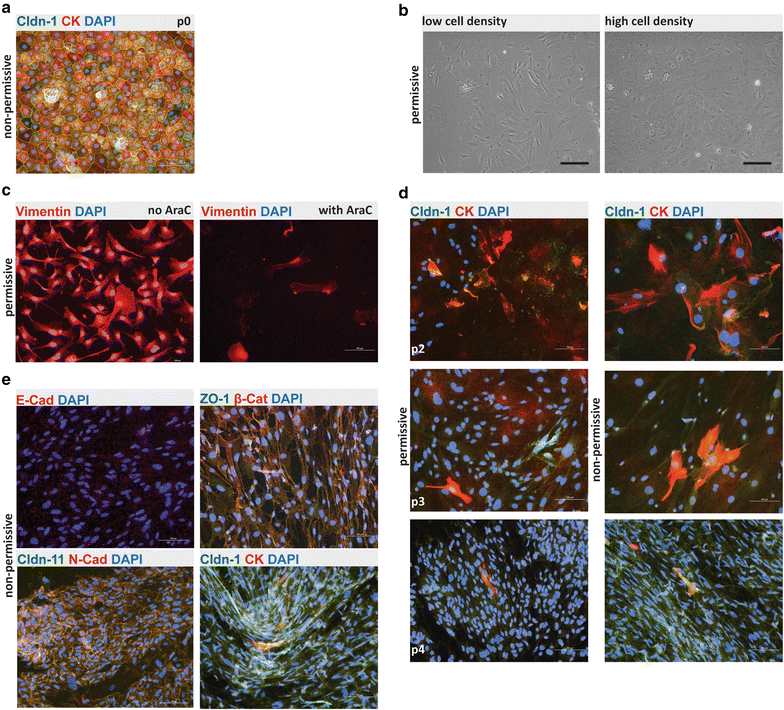
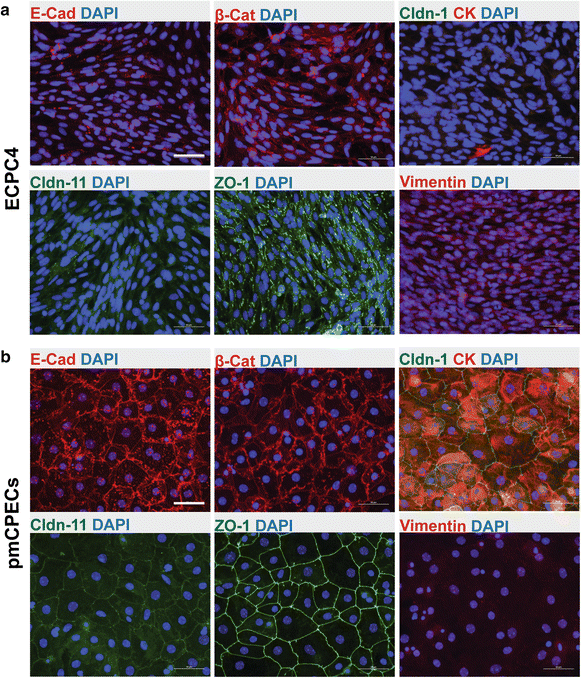
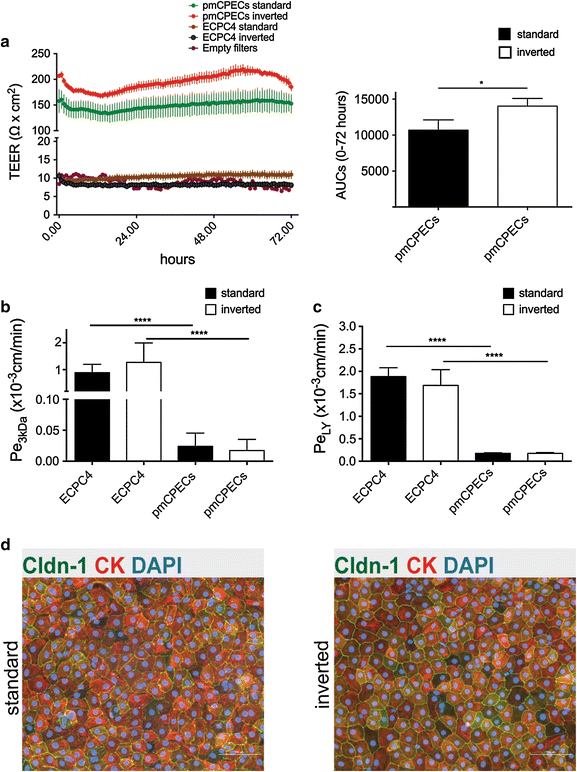
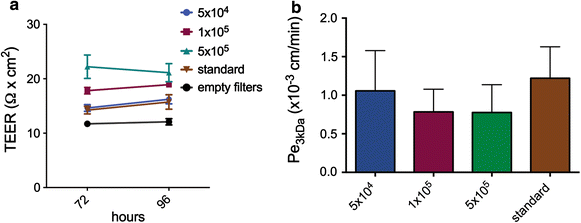
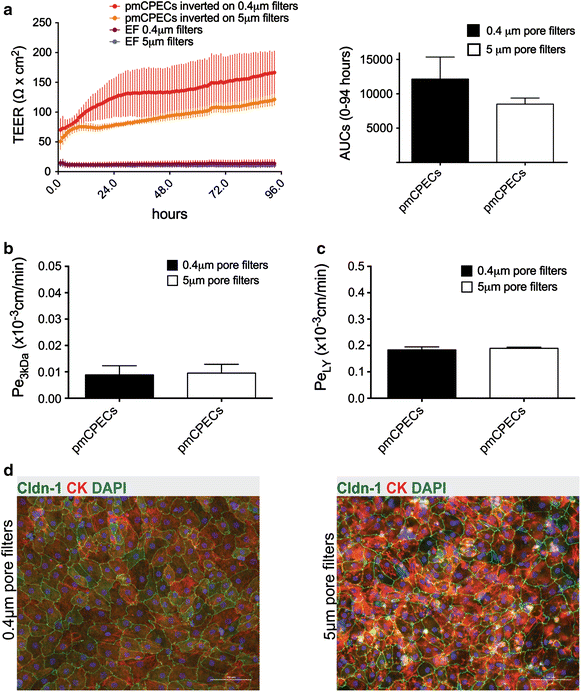
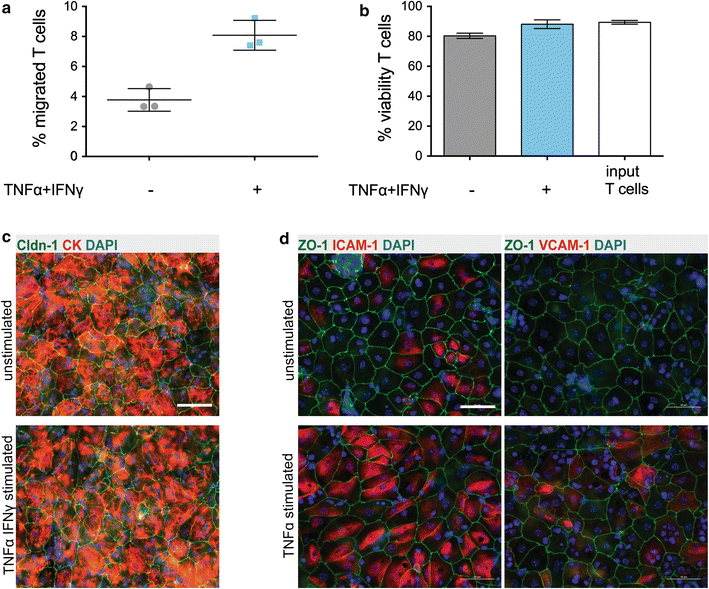
Results: We found that inducible cell line models established from the Immortomouse(®) or the ECPC4 tumor cell line did not express characteristic epithelial proteins such as cytokeratin and E-cadherin and failed to reproducibly establish contact-inhibited epithelial monolayers that formed a tight permeability barrier. In contrast, cultures of highly-purified pmCPECs expressed cytokeratin and displayed mature BCSFB characteristic junctional complexes as visualized by the junctional localization of E-cadherin, β-catenin and claudins-1, -2, -3 and -11. pmCPECs formed a tight barrier with low permeability and high electrical resistance. When grown in inverted filter cultures, pmCPECs were suitable to study T cell migration from the basolateral to the apical side of the BCSFB, thus correctly modelling in vivo migration of immune cells from the blood to the CSF.
Conclusions: Our study excludes inducible and tumor cell line mouse models as suitable to study immune functions of the BCSFB in vitro. Rather, we introduce here an in vitro inverted filter model of the primary mouse BCSFB suited to study the cellular and molecular mechanisms mediating immune cell migration across the BCSFB during immunosurveillance and neuroinflammation.
Figures
Similar articles
-
Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro.Fluids Barriers CNS. 2020 Feb 3;17(1):3. doi: 10.1186/s12987-019-0165-2. Fluids Barriers CNS. 2020. PMID: 32008573 Free PMC article.
-
T-Lymphocytes Traffic into the Brain across the Blood-CSF Barrier: Evidence Using a Reconstituted Choroid Plexus Epithelium.PLoS One. 2016 Mar 4;11(3):e0150945. doi: 10.1371/journal.pone.0150945. eCollection 2016. PLoS One. 2016. PMID: 26942913 Free PMC article.
-
Optimized cultivation of porcine choroid plexus epithelial cells, a blood-cerebrospinal fluid barrier model, for studying granulocyte transmigration.Lab Invest. 2019 Jul;99(8):1245-1255. doi: 10.1038/s41374-019-0250-9. Epub 2019 Apr 17. Lab Invest. 2019. PMID: 30996296
-
A new in vitro model for blood-cerebrospinal fluid barrier transport studies: an immortalized choroid plexus epithelial cell line derived from the tsA58 SV40 large T-antigen gene transgenic rat.Adv Drug Deliv Rev. 2004 Oct 14;56(12):1875-85. doi: 10.1016/j.addr.2004.07.013. Adv Drug Deliv Rev. 2004. PMID: 15381338 Review.
-
Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons.Cytometry A. 2006 Mar;69(3):147-51. doi: 10.1002/cyto.a.20225. Cytometry A. 2006. PMID: 16479603 Review.
Cited by
-
Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro.Fluids Barriers CNS. 2020 Feb 3;17(1):3. doi: 10.1186/s12987-019-0165-2. Fluids Barriers CNS. 2020. PMID: 32008573 Free PMC article.
-
Immune response and pathogen invasion at the choroid plexus in the onset of cerebral toxoplasmosis.J Neuroinflammation. 2022 Jan 13;19(1):17. doi: 10.1186/s12974-021-02370-1. J Neuroinflammation. 2022. PMID: 35027063 Free PMC article.
-
Different Involvement of Vimentin during Invasion by Listeria monocytogenes at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers In Vitro.Int J Mol Sci. 2022 Oct 26;23(21):12908. doi: 10.3390/ijms232112908. Int J Mol Sci. 2022. PMID: 36361697 Free PMC article.
-
The choroid plexus acts as an immune cell reservoir and brain entry site in experimental autoimmune encephalomyelitis.Fluids Barriers CNS. 2023 Jun 1;20(1):39. doi: 10.1186/s12987-023-00441-4. Fluids Barriers CNS. 2023. PMID: 37264368 Free PMC article.
-
Characterization of TRPV4-mediated signaling pathways in an optimized human choroid plexus epithelial cell line.Am J Physiol Cell Physiol. 2022 Dec 1;323(6):C1823-C1842. doi: 10.1152/ajpcell.00193.2022. Epub 2022 Aug 8. Am J Physiol Cell Physiol. 2022. PMID: 35938676 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

