L-Hydroxyproline and d-Proline Catabolism in Sinorhizobium meliloti
- PMID: 26833407
- PMCID: PMC4800863
- DOI: 10.1128/JB.00961-15
L-Hydroxyproline and d-Proline Catabolism in Sinorhizobium meliloti
Abstract
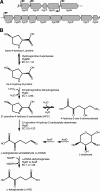
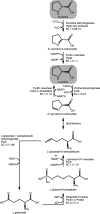
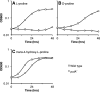
Sinorhizobium meliloti forms N2-fixing root nodules on alfalfa, and as a free-living bacterium, it can grow on a very broad range of substrates, including l-proline and several related compounds, such as proline betaine, trans-4-hydroxy-l-proline (trans-4-l-Hyp), and cis-4-hydroxy-d-proline (cis-4-d-Hyp). Fourteen hyp genes are induced upon growth of S. meliloti on trans-4-l-Hyp, and of those, hypMNPQ encodes an ABC-type trans-4-l-Hyp transporter and hypRE encodes an epimerase that converts trans-4-l-Hyp to cis-4-d-Hyp in the bacterial cytoplasm. Here, we present evidence that the HypO, HypD, and HypH proteins catalyze the remaining steps in which cis-4-d-Hyp is converted to α-ketoglutarate. The HypO protein functions as a d-amino acid dehydrogenase, converting cis-4-d-Hyp to Δ(1)-pyrroline-4-hydroxy-2-carboxylate, which is deaminated by HypD to α-ketoglutarate semialdehyde and then converted to α-ketoglutarate by HypH. The crystal structure of HypD revealed it to be a member of the N-acetylneuraminate lyase subfamily of the (α/β)8 protein family and is consistent with the known enzymatic mechanism for other members of the group. It was also shown that S. meliloti can catabolize d-proline as both a carbon and a nitrogen source, that d-proline can complement l-proline auxotrophy, and that the catabolism of d-proline is dependent on the hyp cluster. Transport of d-proline involves the HypMNPQ transporter, following which d-proline is converted to Δ(1)-pyrroline-2-carboxylate (P2C) largely via HypO. The P2C is converted to l-proline through the NADPH-dependent reduction of P2C by the previously uncharacterized HypS protein. Thus, overall, we have now completed detailed genetic and/or biochemical characterization of 9 of the 14 hyp genes.
Importance: Hydroxyproline is abundant in proteins in animal and plant tissues and serves as a carbon and a nitrogen source for bacteria in diverse environments, including the rhizosphere, compost, and the mammalian gut. While the main biochemical features of bacterial hydroxyproline catabolism were elucidated in the 1960s, the genetic and molecular details have only recently been determined. Elucidating the genetics of hydroxyproline catabolism will aid in the annotation of these genes in other genomes and metagenomic libraries. This will facilitate an improved understanding of the importance of this pathway and may assist in determining the prevalence of hydroxyproline in a particular environment.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Lawrence CC, Sobey WJ, Field RA, Baldwin JE, Schofield CJ. 1996. Purification and initial characterization of proline 4-hydroxylase from Streptomyces griseoviridis P8648: a 2-oxoacid, ferrous-dependent dioxygenase involved in etamycin biosynthesis. Biochem J 313(Part 1):185–191. doi:10.1042/bj3130185. - DOI - PMC - PubMed
-
- Kieliszewski MJ, O'Neill M, Leykam J, Orlando R. 1995. Tandem mass spectrometry and structural elucidation of glycopeptides from a hydroxyproline-rich plant cell wall glycoprotein indicate that contiguous hydroxyproline residues are the major sites of hydroxyproline O-arabinosylation. J Biol Chem 270:2541–2549. doi:10.1074/jbc.270.6.2541. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

