Short-Stalked Prosthecomicrobium hirschii Cells Have a Caulobacter-Like Cell Cycle
- PMID: 26833409
- PMCID: PMC4800870
- DOI: 10.1128/JB.00896-15
Short-Stalked Prosthecomicrobium hirschii Cells Have a Caulobacter-Like Cell Cycle
Abstract
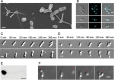
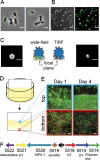
The dimorphic alphaproteobacterium Prosthecomicrobium hirschii has both short-stalked and long-stalked morphotypes. Notably, these morphologies do not arise from transitions in a cell cycle. Instead, the maternal cell morphology is typically reproduced in daughter cells, which results in microcolonies of a single cell type. In this work, we further characterized the short-stalked cells and found that these cells have a Caulobacter-like life cycle in which cell division leads to the generation of two morphologically distinct daughter cells. Using a microfluidic device and total internal reflection fluorescence (TIRF) microscopy, we observed that motile short-stalked cells attach to a surface by means of a polar adhesin. Cells attached at their poles elongate and ultimately release motile daughter cells. Robust biofilm growth occurs in the microfluidic device, enabling the collection of synchronous motile cells and downstream analysis of cell growth and attachment. Analysis of a draft P. hirschii genome sequence indicates the presence of CtrA-dependent cell cycle regulation. This characterization of P. hirschii will enable future studies on the mechanisms underlying complex morphologies and polymorphic cell cycles.
Importance: Bacterial cell shape plays a critical role in regulating important behaviors, such as attachment to surfaces, motility, predation, and cellular differentiation; however, most studies on these behaviors focus on bacteria with relatively simple morphologies, such as rods and spheres. Notably, complex morphologies abound throughout the bacteria, with striking examples, such as P. hirschii, found within the stalked Alphaproteobacteria. P. hirschii is an outstanding candidate for studies of complex morphology generation and polymorphic cell cycles. Here, the cell cycle and genome of P. hirschii are characterized. This work sets the stage for future studies of the impact of complex cell shapes on bacterial behaviors.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

