The Composition of the Cell Envelope Affects Conjugation in Bacillus subtilis
- PMID: 26833415
- PMCID: PMC4859586
- DOI: 10.1128/JB.01044-15
The Composition of the Cell Envelope Affects Conjugation in Bacillus subtilis
Abstract
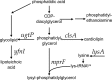
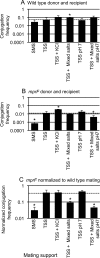
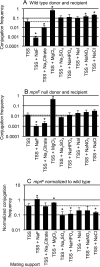
Conjugation in bacteria is the contact-dependent transfer of DNA from one cell to another via donor-encoded conjugation machinery. It is a major type of horizontal gene transfer between bacteria. Conjugation of the integrative and conjugative element ICEBs1 into Bacillus subtilis is affected by the composition of phospholipids in the cell membranes of the donor and recipient. We found that reduction (or elimination) of lysyl-phosphatidylglycerol caused by loss of mpr F caused a decrease in conjugation efficiency. Conversely, alterations that caused an increase in lysyl-phosphatidylglycerol, including loss of ugtP or overproduction of mprF, caused an increase in conjugation efficiency. In addition, we found that mutations that alter production of other phospholipids, e.g., loss of clsA and yfnI, also affected conjugation, apparently without substantively altering levels of lysyl-phosphatidylglycerol, indicating that there are multiple pathways by which changes to the cell envelope affect conjugation. We found that the contribution of mprF to conjugation was affected by the chemical environment. Wild-type cells were generally more responsive to addition of anions that enhanced conjugation, whereas mprF mutant cells were more sensitive to combinations of anions that inhibited conjugation at pH 7. Our results indicate that mprF and lysyl-phosphatidylglycerol allow cells to maintain relatively consistent conjugation efficiencies under a variety of ionic conditions.
Importance: Horizontal gene transfer is a driving force in microbial evolution, enabling cells that receive DNA to acquire new genes and phenotypes. Conjugation, the contact-dependent transfer of DNA from a donor to a recipient by a donor-encoded secretion machine, is a prevalent type of horizontal gene transfer. Although critically important, it is not well understood how the recipient influences the success of conjugation. We found that the composition of phospholipids in the membranes of donors and recipients influences the success of transfer of the integrative and conjugative element ICEBs1 in Bacillus subtilis Specifically, the presence of lysyl-phosphatidylglycerol enables relatively constant conjugation efficiencies in a range of diverse chemical environments.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Critical Components of the Conjugation Machinery of the Integrative and Conjugative Element ICEBs1 of Bacillus subtilis.J Bacteriol. 2015 Aug 1;197(15):2558-67. doi: 10.1128/JB.00142-15. Epub 2015 May 26. J Bacteriol. 2015. PMID: 26013486 Free PMC article.
-
Specificity and Selective Advantage of an Exclusion System in the Integrative and Conjugative Element ICEBs1 of Bacillus subtilis.J Bacteriol. 2021 Apr 21;203(10):e00700-20. doi: 10.1128/JB.00700-20. Print 2021 Apr 21. J Bacteriol. 2021. PMID: 33649151 Free PMC article.
-
Identification of host genes that affect acquisition of an integrative and conjugative element in Bacillus subtilis.Mol Microbiol. 2014 Sep;93(6):1284-301. doi: 10.1111/mmi.12736. Epub 2014 Aug 15. Mol Microbiol. 2014. PMID: 25069588 Free PMC article.
-
Biology of ICEBs1, an integrative and conjugative element in Bacillus subtilis.Plasmid. 2016 Jul;86:14-25. doi: 10.1016/j.plasmid.2016.07.001. Epub 2016 Jul 2. Plasmid. 2016. PMID: 27381852 Review.
-
Diverse regulatory circuits for transfer of conjugative elements.FEMS Microbiol Lett. 2014 Sep;358(2):119-28. doi: 10.1111/1574-6968.12526. Epub 2014 Jul 17. FEMS Microbiol Lett. 2014. PMID: 24995588 Review.
Cited by
-
Genome-Wide Association Study Reveals Host Factors Affecting Conjugation in Escherichia coli.Microorganisms. 2022 Mar 12;10(3):608. doi: 10.3390/microorganisms10030608. Microorganisms. 2022. PMID: 35336183 Free PMC article.
-
Transmating: conjugative transfer of a new broad host range expression vector to various Bacillus species using a single protocol.BMC Microbiol. 2018 Jun 8;18(1):56. doi: 10.1186/s12866-018-1198-4. BMC Microbiol. 2018. PMID: 29884129 Free PMC article.
-
The extended mobility of plasmids.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf652. doi: 10.1093/nar/gkaf652. Nucleic Acids Res. 2025. PMID: 40694848 Free PMC article. Review.
-
Minimization of the Bacillus subtilis divisome suggests FtsZ and SepF can form an active Z-ring, and reveals the amino acid transporter BraB as a new cell division influencing factor.PLoS Genet. 2025 Jan 27;21(1):e1011567. doi: 10.1371/journal.pgen.1011567. eCollection 2025 Jan. PLoS Genet. 2025. PMID: 39869651 Free PMC article.
-
Insights in the Complex DegU, DegS, and Spo0A Regulation System of Paenibacillus polymyxa by CRISPR-Cas9-Based Targeted Point Mutations.Appl Environ Microbiol. 2022 Jun 14;88(11):e0016422. doi: 10.1128/aem.00164-22. Epub 2022 May 19. Appl Environ Microbiol. 2022. PMID: 35588272 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

