Colanic Acid Intermediates Prevent De Novo Shape Recovery of Escherichia coli Spheroplasts, Calling into Question Biological Roles Previously Attributed to Colanic Acid
- PMID: 26833417
- PMCID: PMC4859594
- DOI: 10.1128/JB.01034-15
Colanic Acid Intermediates Prevent De Novo Shape Recovery of Escherichia coli Spheroplasts, Calling into Question Biological Roles Previously Attributed to Colanic Acid
Abstract
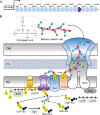
After losing their protective peptidoglycan, bacterial spheroplasts can resynthesize a cell wall to recreate their normal shape. In Escherichia coli, this process requires the Rcs response. In its absence, spheroplasts do not revert to rod shapes but instead form enlarged spheroids and lyse. Here, we investigated the reason for this Rcs requirement. Rcs-deficient spheroids exhibited breaks and bulges in their periplasmic spaces and failed to synthesize a complete peptidoglycan cell wall, indicating that the bacterial envelope was defective. To determine the Rcs-dependent gene(s) required for shape recovery, we tested spheroplasts lacking selected RcsB-regulated genes and found that colanic acid (CA) biosynthesis appeared to be involved. Surprisingly, though, extracellular CA was not required for recovery. Instead, lysis was caused by mutations that interrupted CA biosynthesis downstream of the initial glycosyl transferase, WcaJ. Deleting wcaJ prevented lysis of spheroplasts lacking ensuing steps in the pathway, and providing WcaJ in trans to a mutant lacking the entire CA operon triggered spheroplast enlargement and lysis. Thus, CA is not required for spheroplast recovery. Instead, CA intermediates accumulate as dead-end products which inhibit recovery of wall-less cells. The results strongly imply that CA may not be required for the survival E. coli L-forms. More broadly, these findings mandate that previous conclusions about the role of colanic acid in biofilm formation or virulence must be reevaluated.
Importance: Wall-less bacteria can resynthesize their walls and recreate a normal shape, which in Escherichia coli requires the Rcs response. While attempting to identify the Rcs-dependent gene required for shape recovery, we found that colanic acid (CA) biosynthesis appeared to be involved. Surprisingly, though, cell death was caused by mutations that interrupted CA biosynthesis downstream of the initial step in the pathway, creating dead-end compounds that inhibited recovery of wall-less cells. When testing for the biological role of CA, most previous experiments used mutants that would accumulate these deadly intermediates, meaning that all prior conclusions must be reexamined to determine if the results were caused by these lethal side effects instead of accurately reflecting the biological purpose of CA itself.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
PBP1B Glycosyltransferase and Transpeptidase Activities Play Different Essential Roles during the De Novo Regeneration of Rod Morphology in Escherichia coli.J Bacteriol. 2017 Mar 14;199(7):e00612-16. doi: 10.1128/JB.00612-16. Print 2017 Apr 1. J Bacteriol. 2017. PMID: 28096447 Free PMC article.
-
The Rcs stress response and accessory envelope proteins are required for de novo generation of cell shape in Escherichia coli.J Bacteriol. 2013 Jun;195(11):2452-62. doi: 10.1128/JB.00160-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543719 Free PMC article.
-
The Rcs-Regulated Colanic Acid Capsule Maintains Membrane Potential in Salmonella enterica serovar Typhimurium.mBio. 2017 Jun 6;8(3):e00808-17. doi: 10.1128/mBio.00808-17. mBio. 2017. PMID: 28588134 Free PMC article.
-
Nucleotide sequence of rmpB, a Klebsiella pneumoniae gene that positively controls, colanic biosynthesis in Escherichia coli.Res Microbiol. 1991 Jan;142(1):47-54. doi: 10.1016/0923-2508(91)90096-s. Res Microbiol. 1991. PMID: 2068379 Review.
-
Factors That Affect the Enlargement of Bacterial Protoplasts and Spheroplasts.Int J Mol Sci. 2020 Sep 27;21(19):7131. doi: 10.3390/ijms21197131. Int J Mol Sci. 2020. PMID: 32992574 Free PMC article. Review.
Cited by
-
Interrupting Biosynthesis of O Antigen or the Lipopolysaccharide Core Produces Morphological Defects in Escherichia coli by Sequestering Undecaprenyl Phosphate.J Bacteriol. 2016 Oct 21;198(22):3070-3079. doi: 10.1128/JB.00550-16. Print 2016 Nov 15. J Bacteriol. 2016. PMID: 27573014 Free PMC article.
-
The Determination, Monitoring, Molecular Mechanisms and Formation of Biofilm in E. coli.Braz J Microbiol. 2023 Mar;54(1):259-277. doi: 10.1007/s42770-022-00895-y. Epub 2022 Dec 29. Braz J Microbiol. 2023. PMID: 36577889 Free PMC article. Review.
-
General Utilization of Fluorescent Polyisoprenoids with Sugar Selective Phosphoglycosyltransferases.Biochemistry. 2020 Feb 4;59(4):615-626. doi: 10.1021/acs.biochem.9b01026. Epub 2020 Jan 7. Biochemistry. 2020. PMID: 31876413 Free PMC article.
-
Escherichia coli adaptation and response to exposure to heavy atmospheric pollution.Sci Rep. 2019 Jul 26;9(1):10879. doi: 10.1038/s41598-019-47427-7. Sci Rep. 2019. PMID: 31350435 Free PMC article.
-
The O-Antigen Flippase Wzk Can Substitute for MurJ in Peptidoglycan Synthesis in Helicobacter pylori and Escherichia coli.PLoS One. 2016 Aug 18;11(8):e0161587. doi: 10.1371/journal.pone.0161587. eCollection 2016. PLoS One. 2016. PMID: 27537185 Free PMC article.
References
-
- Wang S, Shaevitz JW. 2013. The mechanics of shape in prokaryotes. Front Biosci (Schol Ed) 5:564–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

