Different Functions of Phylogenetically Distinct Bacterial Complex I Isozymes
- PMID: 26833419
- PMCID: PMC4859585
- DOI: 10.1128/JB.01025-15
Different Functions of Phylogenetically Distinct Bacterial Complex I Isozymes
Abstract
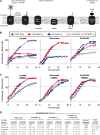
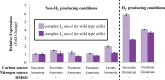
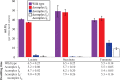
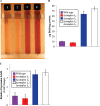
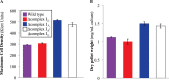
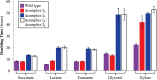
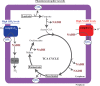
NADH:quinone oxidoreductase (complex I) is a bioenergetic enzyme that transfers electrons from NADH to quinone, conserving the energy of this reaction by contributing to the proton motive force. While the importance of NADH oxidation to mitochondrial aerobic respiration is well documented, the contribution of complex I to bacterial electron transport chains has been tested in only a few species. Here, we analyze the function of two phylogenetically distinct complex I isozymes in Rhodobacter sphaeroides, an alphaproteobacterium that contains well-characterized electron transport chains. We found that R. sphaeroides complex I activity is important for aerobic respiration and required for anaerobic dimethyl sulfoxide (DMSO) respiration (in the absence of light), photoautotrophic growth, and photoheterotrophic growth (in the absence of an external electron acceptor). Our data also provide insight into the functions of the phylogenetically distinct R. sphaeroidescomplex I enzymes (complex IA and complex IE) in maintaining a cellular redox state during photoheterotrophic growth. We propose that the function of each isozyme during photoheterotrophic growth is either NADH synthesis (complex IA) or NADH oxidation (complex IE). The canonical alphaproteobacterial complex I isozyme (complex IA) was also shown to be important for routing electrons to nitrogenase-mediated H2 production, while the horizontally acquired enzyme (complex IE) was dispensable in this process. Unlike the singular role of complex I in mitochondria, we predict that the phylogenetically distinct complex I enzymes found across bacterial species have evolved to enhance the functions of their respective electron transport chains.
Importance: Cells use a proton motive force (PMF), NADH, and ATP to support numerous processes. In mitochondria, complex I uses NADH oxidation to generate a PMF, which can drive ATP synthesis. This study analyzed the function of complex I in bacteria, which contain more-diverse and more-flexible electron transport chains than mitochondria. We tested complex I function in Rhodobacter sphaeroides, a bacterium predicted to encode two phylogenetically distinct complex I isozymes. R. sphaeroides cells lacking both isozymes had growth defects during all tested modes of growth, illustrating the important function of this enzyme under diverse conditions. We conclude that the two isozymes are not functionally redundant and predict that phylogenetically distinct complex I enzymes have evolved to support the diverse lifestyles of bacteria.
Copyright © 2016 Spero et al.
Figures

Similar articles
-
Phylogenomic analysis and predicted physiological role of the proton-translocating NADH:quinone oxidoreductase (complex I) across bacteria.mBio. 2015 Apr 14;6(2):e00389-15. doi: 10.1128/mBio.00389-15. mBio. 2015. PMID: 25873378 Free PMC article.
-
Genetic and Biochemical Analysis of Anaerobic Respiration in Bacteroides fragilis and Its Importance In Vivo.mBio. 2020 Feb 4;11(1):e03238-19. doi: 10.1128/mBio.03238-19. mBio. 2020. PMID: 32019804 Free PMC article.
-
Engineering the transcriptional activator NifA for the construction of Rhodobacter sphaeroides strains that produce hydrogen gas constitutively.Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9739-9749. doi: 10.1007/s00253-019-10199-1. Epub 2019 Nov 7. Appl Microbiol Biotechnol. 2019. PMID: 31696284
-
Photosynthetic electron transport and anaerobic metabolism in purple non-sulfur phototrophic bacteria.Antonie Van Leeuwenhoek. 1994;66(1-3):151-64. doi: 10.1007/BF00871637. Antonie Van Leeuwenhoek. 1994. PMID: 7747929 Review.
-
Five decades of research on mitochondrial NADH-quinone oxidoreductase (complex I).Biol Chem. 2018 Oct 25;399(11):1249-1264. doi: 10.1515/hsz-2018-0164. Biol Chem. 2018. PMID: 30243012 Review.
Cited by
-
Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira.ISME J. 2018 Jun;12(7):1779-1793. doi: 10.1038/s41396-018-0083-3. Epub 2018 Mar 7. ISME J. 2018. PMID: 29515170 Free PMC article.
-
Mechanisms for Generating Low Potential Electrons across the Metabolic Diversity of Nitrogen-Fixing Bacteria.Appl Environ Microbiol. 2023 May 31;89(5):e0037823. doi: 10.1128/aem.00378-23. Epub 2023 May 8. Appl Environ Microbiol. 2023. PMID: 37154716 Free PMC article. Review.
-
Combining Genome-Scale Experimental and Computational Methods To Identify Essential Genes in Rhodobacter sphaeroides.mSystems. 2017 Jun 6;2(3):e00015-17. doi: 10.1128/mSystems.00015-17. eCollection 2017 May-Jun. mSystems. 2017. PMID: 28744485 Free PMC article.
-
Syntrophic interspecies electron transfer drives carbon fixation and growth by Rhodopseudomonas palustris under dark, anoxic conditions.Sci Adv. 2021 Jul 2;7(27):eabh1852. doi: 10.1126/sciadv.abh1852. Print 2021 Jul. Sci Adv. 2021. PMID: 34215588 Free PMC article.
-
Tracking Electron Uptake from a Cathode into Shewanella Cells: Implications for Energy Acquisition from Solid-Substrate Electron Donors.mBio. 2018 Feb 27;9(1):e02203-17. doi: 10.1128/mBio.02203-17. mBio. 2018. PMID: 29487241 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

