Cholesterol Analogs with Degradation-resistant Alkyl Side Chains Are Effective Mycobacterium tuberculosis Growth Inhibitors
- PMID: 26833565
- PMCID: PMC4817165
- DOI: 10.1074/jbc.M115.708172
Cholesterol Analogs with Degradation-resistant Alkyl Side Chains Are Effective Mycobacterium tuberculosis Growth Inhibitors
Abstract
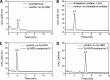
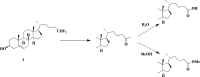
Cholest-4-en-3-one, whether added exogenously or generated intracellularly from cholesterol, inhibits the growth ofMycobacterium tuberculosiswhen CYP125A1 and CYP142A1, the cytochrome P450 enzymes that initiate degradation of the sterol side chain, are disabled. Here we demonstrate that a 16-hydroxy derivative of cholesterol, which was previously reported to inhibit growth ofM. tuberculosis, acts by preventing the oxidation of the sterol side chain even in the presence of the relevant cytochrome P450 enzymes. The finding that (25R)-cholest-5-en-3β,16β,26-triol (1) (and its 3-keto metabolite) inhibit growth suggests that cholesterol analogs with non-degradable side chains represent a novel class of anti-mycobacterial agents. In accord with this, two cholesterol analogs with truncated, fluorinated side chains have been synthesized and shown to similarly block the growth in culture ofM. tuberculosis.
Keywords: Mycobacterium tuberculosis; cholesterol metabolism; cytochrome P450; dehydrogenase; enzyme inhibitor.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


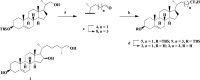


References
-
- World Health Organization (2015) Tuberculosis, Fact Sheet No. 104, World Health Organization, http://www.who.int/mediacentre/factsheets/fs104/en/
-
- Sharma S. K., and Mohan A. (2006) Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 130, 261–272 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

