Multiple Domains of GlcNAc-1-phosphotransferase Mediate Recognition of Lysosomal Enzymes
- PMID: 26833567
- PMCID: PMC4825028
- DOI: 10.1074/jbc.M116.714568
Multiple Domains of GlcNAc-1-phosphotransferase Mediate Recognition of Lysosomal Enzymes
Abstract
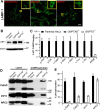
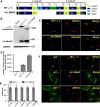
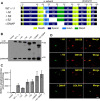
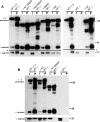
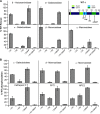
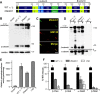
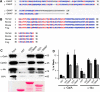
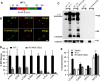
The Golgi enzyme UDP-GlcNAc:lysosomal enzymeN-acetylglucosamine-1-phosphotransferase (GlcNAc-1-phosphotransferase), an α2β2γ2hexamer, mediates the initial step in the addition of the mannose 6-phosphate targeting signal on newly synthesized lysosomal enzymes. This tag serves to direct the lysosomal enzymes to lysosomes. A key property of GlcNAc-1-phosphotransferase is its unique ability to distinguish the 60 or so lysosomal enzymes from the numerous non-lysosomal glycoproteins with identical Asn-linked glycans. In this study, we demonstrate that the two Notch repeat modules and the DNA methyltransferase-associated protein interaction domain of the α subunit are key components of this recognition process. Importantly, different combinations of these domains are involved in binding to individual lysosomal enzymes. This study also identifies the γ-binding site on the α subunit and demonstrates that in the majority of instances the mannose 6-phosphate receptor homology domain of the γ subunit is required for optimal phosphorylation. These findings serve to explain how GlcNAc-1-phosphotransferase recognizes a large number of proteins that lack a common structural motif.
Keywords: lysosomal glycoprotein; lysosomal storage disease; lysosome; mannose 6-phosphate (Man-6-P); phosphorylation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Reitman M. L., and Kornfeld S. (1981) Lysosomal enzyme targeting. N-Acetylglucosaminylphosphotransferase selectively phosphorylates native lysosomal enzymes. J. Biol. Chem. 256, 11977–11980 - PubMed
-
- Varki A., and Kornfeld S. (1980) Identification of a rat liver α-N-acetylglucosaminyl phosphodiesterase capable of removing “blocking” α-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J. Biol. Chem. 255, 8398–8401 - PubMed
-
- Bao M., Booth J. L., Elmendorf B. J., and Canfield W. M. (1996) Bovine UDP-N-acetylglucosamine:lysosomal-enzyme N-acetylglucosamine-1-phosphotransferase. I. Purification and subunit structure. J. Biol. Chem. 271, 31437–31445 - PubMed
-
- Kudo M., Bao M., D'Souza A., Ying F., Pan H., Roe B. A., and Canfield W. M. (2005) The α- and β-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J. Biol. Chem. 280, 36141–36149 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

