Exposure-response analyses of liraglutide 3.0 mg for weight management
- PMID: 26833744
- PMCID: PMC5069568
- DOI: 10.1111/dom.12639
Exposure-response analyses of liraglutide 3.0 mg for weight management
Abstract
Aims: Liraglutide 3.0 mg, an acylated GLP-1 analogue approved for weight management, lowers body weight through decreased energy intake. We conducted exposure-response analyses to provide important information on individual responses to given drug doses, reflecting inter-individual variations in drug metabolism, absorption and excretion.
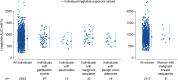
Methods: We report efficacy and safety responses across a wide range of exposure levels, using data from one phase II (liraglutide doses 1.2, 1.8, 2.4 and 3.0 mg), and two phase IIIa [SCALE Obesity and Prediabetes (3.0 mg); SCALE Diabetes (1.8; 3.0 mg)] randomized, placebo-controlled trials (n = 4372).
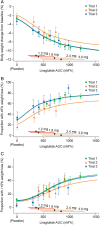
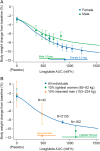
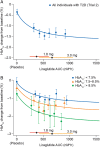
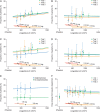
Results: There was a clear exposure-weight loss response. Weight loss increased with greater exposure and appeared to level off at the highest exposures associated with liraglutide 3.0 mg in most individuals, but did not fully plateau in men. In individuals with overweight/obesity and comorbid type 2 diabetes, there was a clear exposure-glycated haemoglobin (HbA1c) relationship. HbA1c reduction increased with higher plasma liraglutide concentration (plateauing at ∼21 nM); however, for individuals with baseline HbA1c >8.5%, HbA1c reduction did not fully plateau. No exposure-response relationship was identified for any safety outcome, with the exception of gastrointestinal adverse events (AEs). Individuals with gallbladder AEs, acute pancreatitis or malignant/breast/benign colorectal neoplasms did not have higher liraglutide exposure compared with the overall population.
Conclusions: These analyses support the use of liraglutide 3.0 mg for weight management in all subgroups investigated; weight loss increased with higher drug exposure, with no concomitant deterioration in safety/tolerability besides previously known gastrointestinal side effects.
Keywords: body weight; glucagon-like peptide-1; incretin; pharmacokinetic.
© 2016 John Wiley & Sons Ltd.
Figures
References
-
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP‐1 binding sites in the rat brain: evidence that exendin‐4 is a ligand of brain GLP‐1 binding sites. Eur J Neurosci 1995; 7: 2294–2300. - PubMed
-
- Turton MD, O'Shea D, Gunn I et al. A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 1996; 379: 69–72. - PubMed
-
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403: 261–280. - PubMed
-
- Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon‐like peptide‐1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001; 25: 781–792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

