Dynamics of the actin cytoskeleton mediates receptor cross talk: An emerging concept in tuning receptor signaling
- PMID: 26833785
- PMCID: PMC4748574
- DOI: 10.1083/jcb.201504137
Dynamics of the actin cytoskeleton mediates receptor cross talk: An emerging concept in tuning receptor signaling
Abstract
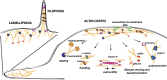
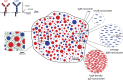
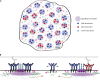
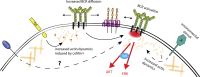
Recent evidence implicates the actin cytoskeleton in the control of receptor signaling. This may be of particular importance in the context of immune receptors, such as the B cell receptor, where dysregulated signaling can result in autoimmunity and malignancy. Here, we discuss the role of the actin cytoskeleton in controlling receptor compartmentalization, dynamics, and clustering as a means to regulate receptor signaling through controlling the interactions with protein partners. We propose that the actin cytoskeleton is a point of integration for receptor cross talk through modulation of protein dynamics and clustering. We discuss the implication of this cross talk via the cytoskeleton for both ligand-induced and low-level constitutive (tonic) signaling necessary for immune cell survival.
© 2016 Mattila et al.
Figures

Similar articles
-
GPCRs and actin-cytoskeleton dynamics.Methods Cell Biol. 2016;132:165-88. doi: 10.1016/bs.mcb.2015.10.003. Epub 2016 Feb 10. Methods Cell Biol. 2016. PMID: 26928544
-
Phagocytosis: receptors, signal integration, and the cytoskeleton.Immunol Rev. 2014 Nov;262(1):193-215. doi: 10.1111/imr.12212. Immunol Rev. 2014. PMID: 25319336 Review.
-
B cell receptor-induced Ca2+ mobilization mediates F-actin rearrangements and is indispensable for adhesion and spreading of B lymphocytes.J Leukoc Biol. 2013 Apr;93(4):537-47. doi: 10.1189/jlb.0312169. Epub 2013 Jan 29. J Leukoc Biol. 2013. PMID: 23362305
-
Signaling to Rho GTPases.Exp Cell Res. 1999 Nov 25;253(1):166-79. doi: 10.1006/excr.1999.4674. Exp Cell Res. 1999. PMID: 10579921 Review.
-
Cytoskeletal cross-talk in the control of T cell antigen receptor signaling.FEBS Lett. 2010 Dec 15;584(24):4845-50. doi: 10.1016/j.febslet.2010.09.001. Epub 2010 Sep 7. FEBS Lett. 2010. PMID: 20828561 Review.
Cited by
-
Molecular basis for potent B cell responses to antigen displayed on particles of viral size.Nat Immunol. 2023 Oct;24(10):1762-1777. doi: 10.1038/s41590-023-01597-9. Epub 2023 Aug 31. Nat Immunol. 2023. PMID: 37653247 Free PMC article.
-
The Lack of WIP Binding to Actin Results in Impaired B Cell Migration and Altered Humoral Immune Responses.Cell Rep. 2018 Jul 17;24(3):619-629. doi: 10.1016/j.celrep.2018.06.051. Cell Rep. 2018. PMID: 30021160 Free PMC article.
-
Differential gene expression analysis by RNA-seq reveals the importance of actin cytoskeletal proteins in erythroleukemia cells.PeerJ. 2017 Jun 27;5:e3432. doi: 10.7717/peerj.3432. eCollection 2017. PeerJ. 2017. PMID: 28663935 Free PMC article.
-
Molecular association of CD98, CD29, and CD147 critically mediates monocytic U937 cell adhesion.Korean J Physiol Pharmacol. 2016 Sep;20(5):515-23. doi: 10.4196/kjpp.2016.20.5.515. Epub 2016 Aug 26. Korean J Physiol Pharmacol. 2016. PMID: 27610038 Free PMC article.
-
Mechanically active integrins target lytic secretion at the immune synapse to facilitate cellular cytotoxicity.Nat Commun. 2022 Jun 9;13(1):3222. doi: 10.1038/s41467-022-30809-3. Nat Commun. 2022. PMID: 35680882 Free PMC article.
References
-
- Arana E., Vehlow A., Harwood N.E., Vigorito E., Henderson R., Turner M., Tybulewicz V.L., and Batista F.D.. 2008. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 28:88–99. 10.1016/j.immuni.2007.12.003 - DOI - PubMed
-
- Avalos A.M., Bilate A.M., Witte M.D., Tai A.K., He J., Frushicheva M.P., Thill P.D., Meyer-Wentrup F., Theile C.S., Chakraborty A.K., et al. . 2014. Monovalent engagement of the BCR activates ovalbumin-specific transnuclear B cells. J. Exp. Med. 211:365–379. 10.1084/jem.20131603 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

