Epistasis in protein evolution
- PMID: 26833806
- PMCID: PMC4918427
- DOI: 10.1002/pro.2897
Epistasis in protein evolution
Abstract
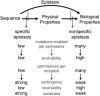
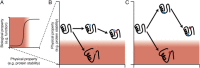
The structure, function, and evolution of proteins depend on physical and genetic interactions among amino acids. Recent studies have used new strategies to explore the prevalence, biochemical mechanisms, and evolutionary implications of these interactions-called epistasis-within proteins. Here we describe an emerging picture of pervasive epistasis in which the physical and biological effects of mutations change over the course of evolution in a lineage-specific fashion. Epistasis can restrict the trajectories available to an evolving protein or open new paths to sequences and functions that would otherwise have been inaccessible. We describe two broad classes of epistatic interactions, which arise from different physical mechanisms and have different effects on evolutionary processes. Specific epistasis-in which one mutation influences the phenotypic effect of few other mutations-is caused by direct and indirect physical interactions between mutations, which nonadditively change the protein's physical properties, such as conformation, stability, or affinity for ligands. In contrast, nonspecific epistasis describes mutations that modify the effect of many others; these typically behave additively with respect to the physical properties of a protein but exhibit epistasis because of a nonlinear relationship between the physical properties and their biological effects, such as function or fitness. Both types of interaction are rampant, but specific epistasis has stronger effects on the rate and outcomes of evolution, because it imposes stricter constraints and modulates evolutionary potential more dramatically; it therefore makes evolution more contingent on low-probability historical events and leaves stronger marks on the sequences, structures, and functions of protein families.
Keywords: ancestral sequence reconstruction; deep mutational scanning; epistasis; evolutionary biochemistry; protein evolution; sequence space; sequence-function relationship.
© 2016 The Protein Society.
Figures


References
-
- Breen MS, Kemena C, Vlasov PK, Notredame C, Kondrashov FA (2012) Epistasis as the primary factor in molecular evolution. Nature 490:535–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

