Epigenetic mechanisms in sexual differentiation of the brain and behaviour
- PMID: 26833835
- PMCID: PMC4785900
- DOI: 10.1098/rstb.2015.0114
Epigenetic mechanisms in sexual differentiation of the brain and behaviour
Abstract
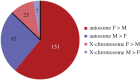
Circumstantial evidence alone argues that the establishment and maintenance of sex differences in the brain depend on epigenetic modifications of chromatin structure. More direct evidence has recently been obtained from two types of studies: those manipulating a particular epigenetic mechanism, and those examining the genome-wide distribution of specific epigenetic marks. The manipulation of histone acetylation or DNA methylation disrupts the development of several neural sex differences in rodents. Taken together, however, the evidence suggests there is unlikely to be a simple formula for masculine or feminine development of the brain and behaviour; instead, underlying epigenetic mechanisms may vary by brain region or even by dependent variable within a region. Whole-genome studies related to sex differences in the brain have only very recently been reported, but suggest that males and females may use different combinations of epigenetic modifications to control gene expression, even in cases where gene expression does not differ between the sexes. Finally, recent findings are discussed that are likely to direct future studies on the role of epigenetic mechanisms in sexual differentiation of the brain and behaviour.
Keywords: DNA methylation; bed nucleus of the stria terminalis; histone; medial preoptic area; sex difference.
© 2016 The Author(s).
Figures
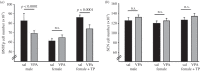



Similar articles
-
Past, present and future of epigenetics in brain sexual differentiation.J Neuroendocrinol. 2018 Feb;30(2). doi: 10.1111/jne.12492. J Neuroendocrinol. 2018. PMID: 28585265 Review.
-
Epigenetic mechanisms are involved in sexual differentiation of the brain.Rev Endocr Metab Disord. 2012 Sep;13(3):163-71. doi: 10.1007/s11154-012-9202-z. Rev Endocr Metab Disord. 2012. PMID: 22327342 Review.
-
Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice.Exp Neurol. 2015 Jun;268:21-9. doi: 10.1016/j.expneurol.2014.08.006. Epub 2014 Aug 14. Exp Neurol. 2015. PMID: 25131640 Free PMC article. Review.
-
Epigenetic contributions to hormonally-mediated sexual differentiation of the brain.J Neuroendocrinol. 2013 Nov;25(11):1133-40. doi: 10.1111/jne.12072. J Neuroendocrinol. 2013. PMID: 23919286 Free PMC article. Review.
-
The potential role of SRY in epigenetic gene regulation during brain sexual differentiation in mammals.Adv Genet. 2014;86:135-65. doi: 10.1016/B978-0-12-800222-3.00007-3. Adv Genet. 2014. PMID: 25172349 Review.
Cited by
-
Sex-specific DNA methylation: impact on human health and development.Mol Genet Genomics. 2022 Nov;297(6):1451-1466. doi: 10.1007/s00438-022-01935-w. Epub 2022 Aug 15. Mol Genet Genomics. 2022. PMID: 35969270 Review.
-
Interplay between prenatal bisphenol exposure, postnatal maternal care, and offspring sex in predicting DNA methylation relevant to anxiety-like behavior in rats.Horm Behav. 2025 Jun;172:105745. doi: 10.1016/j.yhbeh.2025.105745. Epub 2025 Apr 23. Horm Behav. 2025. PMID: 40273582
-
Sexually Dimorphic Formation of the Preoptic Area and the Bed Nucleus of the Stria Terminalis by Neuroestrogens.Front Neurosci. 2020 Jul 29;14:797. doi: 10.3389/fnins.2020.00797. eCollection 2020. Front Neurosci. 2020. PMID: 32848568 Free PMC article. Review.
-
Biological Sex, Estradiol and Striatal Medium Spiny Neuron Physiology: A Mini-Review.Front Cell Neurosci. 2018 Dec 12;12:492. doi: 10.3389/fncel.2018.00492. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618639 Free PMC article. Review.
-
Detection Algorithms for Simple Two-Group Comparisons Using Spontaneous Reporting Systems.Drug Saf. 2024 Jun;47(6):535-543. doi: 10.1007/s40264-024-01404-w. Epub 2024 Feb 22. Drug Saf. 2024. PMID: 38388828 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

