Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer
- PMID: 26834066
- PMCID: PMC4872323
- DOI: 10.1200/JCO.2015.63.0970
Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer
Abstract
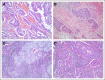
Purpose: Tumor lymphocytic infiltration (TLI) has differing prognostic value among various cancers. The objective of this study was to assess the effect of TLI in lung cancer.
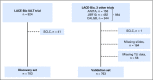
Patients and methods: A discovery set (one trial, n = 824) and a validation set (three trials, n = 984) that evaluated the benefit of platinum-based adjuvant chemotherapy in non-small-cell lung cancer were used as part of the LACE-Bio (Lung Adjuvant Cisplatin Evaluation Biomarker) study. TLI was defined as intense versus nonintense. The main end point was overall survival (OS); secondary end points were disease-free survival (DFS) and specific DFS (SDFS). Hazard ratios (HRs) and 95% CIs associated with TLI were estimated through a multivariable Cox model in both sets. TLI-histology and TLI-treatment interactions were explored in the combined set.
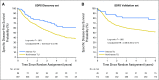
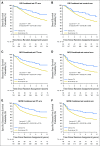
Results: Discovery and validation sets with complete data included 783 (409 deaths) and 763 (344 deaths) patients, respectively. Median follow-up was 4.8 and 6 years, respectively. TLI was intense in 11% of patients in the discovery set compared with 6% in the validation set (P < .001). The prognostic value of TLI in the discovery set (OS: HR, 0.56; 95% CI, 0.38 to 0.81; P = .002; DFS: HR, 0.59; 95% CI, 0.42 to 0.83; P = .002; SDFS: HR, 0.56; 95% CI, 0.38 to 0.82; P = .003) was confirmed in the validation set (OS: HR, 0.45; 95% CI, 0.23 to 0.85; P = .01; DFS: HR, 0.44; 95% CI, 0.24 to 0.78; P = .005; SDFS: HR, 0.42; 95% CI, 0.22 to 0.80; P = .008) with no heterogeneity across trials (P ≥ .38 for all end points). No significant predictive effect was observed for TLI (P ≥ .78 for all end points).
Conclusion: Intense lymphocytic infiltration, found in a minority of tumors, was validated as a favorable prognostic marker for survival in resected non-small-cell lung cancer.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Is the regulation of SIRT1 by miRNA-34a the key to mesenchymal stem cell survival?Ann Transl Med. 2016 Jun;4(12):243. doi: 10.21037/atm.2016.05.45. Ann Transl Med. 2016. PMID: 27428754 Free PMC article. No abstract available.
-
Tumor infiltrating lymphocytes in lung cancer: a new prognostic parameter.J Thorac Dis. 2016 Aug;8(8):E833-5. doi: 10.21037/jtd.2016.07.75. J Thorac Dis. 2016. PMID: 27618931 Free PMC article. No abstract available.
-
Tumor lymphocytic infiltration in non-small cell lung cancer: the ultimate prognostic marker?Transl Lung Cancer Res. 2016 Aug;5(4):370-2. doi: 10.21037/tlcr.2016.07.07. Transl Lung Cancer Res. 2016. PMID: 27650724 Free PMC article. No abstract available.
References
-
- Kataki A, Scheid P, Piet M, et al. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med. 2002;140:320–328. - PubMed
-
- Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. discussion 371-372. - PubMed
-
- Johnson SK, Kerr KM, Chapman AD, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

