Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone
- PMID: 26834067
- PMCID: PMC4966514
- DOI: 10.1200/JCO.2015.64.0821
Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone
Abstract
Purpose: Transarterial chemoembolization is accepted therapy for hepatocellular carcinoma (HCC). No randomized trial has demonstrated superiority of chemoembolization compared with embolization, and the role of chemotherapy remains unclear. This randomized trial compares the outcome of embolization using microspheres alone with chemoembolization using doxorubicin-eluting microspheres.
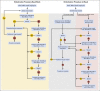
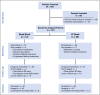
Materials and methods: At a single tertiary referral center, patients with HCC were randomly assigned to embolization with microspheres alone (Bead Block [BB]) or loaded with doxorubicin 150 mg (LC Bead [LCB]). Random assignment was stratified by number of embolizations to complete treatment, and assignments were generated by permuted blocks in the institutional database. The primary end point was response according to RECIST 1.0 (Response Evaluation Criteria in Solid Tumors) using multiphase computed tomography 2 to 3 weeks post-treatment and then at quarterly intervals, with the reviewer blinded to treatment allocation. Secondary objectives included safety and tolerability, time to progression, progression-free survival, and overall survival. This trial is currently closed to accrual.
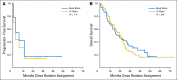
Results: Between December 2007 and April 2012, 101 patients were randomly assigned: 51 to BB and 50 to LCB. Demographics were comparable: median age, 67 years; 77% male; and 22% Barcelona Clinic Liver Cancer stage A and 78% stage B or C. Adverse events occurred with similar frequency in both groups: BB, 19 of 51 patients (38%); LCB, 20 of 50 patients (40%; P = .48), with no difference in RECIST response: BB, 5.9% versus LCB, 6.0% (difference, -0.1%; 95% CI, -9% to 9%). Median PFS was 6.2 versus 2.8 months (hazard ratio, 1.36; 95% CI, 0.91 to 2.05; P = .11), and overall survival, 19.6 versus 20.8 months (hazard ratio, 1.11; 95% CI, 0.71 to 1.76; P = .64) for BB and LCB, respectively.
Conclusion: There was no apparent difference between the treatment arms. These results challenge the use of doxorubicin-eluting beads for chemoembolization of HCC.
Trial registration: ClinicalTrials.gov NCT00539643.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Delusional Beliefs and Hepatocellular Carcinoma.J Clin Oncol. 2017 Jan 10;35(2):256. doi: 10.1200/JCO.2016.67.2337. Epub 2016 Oct 23. J Clin Oncol. 2017. PMID: 28056192 No abstract available.
-
Chemoembolization or Bland Embolization for Hepatocellular Carcinoma: The Question Is Still Unanswered.J Clin Oncol. 2017 Jan 10;35(2):256-257. doi: 10.1200/JCO.2016.67.2915. Epub 2016 Oct 23. J Clin Oncol. 2017. PMID: 28056196 No abstract available.
-
Transarterial Embolization With or Without Chemotherapy: What Should Be the Indication for Patients With Hepatocellular Carcinoma?J Clin Oncol. 2017 Jan 10;35(2):257-258. doi: 10.1200/JCO.2016.68.4902. Epub 2016 Oct 23. J Clin Oncol. 2017. PMID: 28056197 No abstract available.
-
Reply to A. Braillon, M. Boulin et al, and J.-H. Zhong et al.J Clin Oncol. 2017 Jan 10;35(2):258-259. doi: 10.1200/JCO.2016.69.7961. Epub 2016 Oct 23. J Clin Oncol. 2017. PMID: 28056199 No abstract available.
References
-
- Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet. 2002;359:1734–1739. - PubMed
-
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. - PubMed
-
- Brown DB, Geschwind JF, Soulen MC, et al. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2006;17:217–223. - PubMed
-
- Bruix J, Sherman M, Llovet JM, et al. EASL Panel of Experts on HCC. European Association for the Study of the Liver Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

