Performance of Interferon-Gamma and IP-10 Release Assays for Diagnosing Latent Tuberculosis Infections in Patients with Concurrent Malaria in Tanzania
- PMID: 26834199
- PMCID: PMC4824211
- DOI: 10.4269/ajtmh.15-0633
Performance of Interferon-Gamma and IP-10 Release Assays for Diagnosing Latent Tuberculosis Infections in Patients with Concurrent Malaria in Tanzania
Abstract
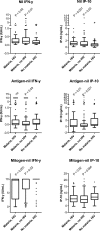
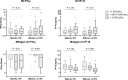
Interferon-gamma (IFN-γ) release assays (IGRAs) are used to detect cellular immune recognition of Mycobacterium tuberculosis The chemokine IFN-γ-inducible protein 10 (IP-10) is an alternative diagnostic biomarker to IFN-γ. Several conditions interfere with IGRA test performance. We aimed to assess the possible influence of Plasmodium falciparum infection on the IGRA test QuantiFERON-TB GOLD® In-Tube (QFT) test and an in-house IP-10 release assay. In total, 241 Tanzanian adults were included; 184 patients with uncomplicated malaria (88 human immunodeficiency virus [HIV] coinfected) and 57 HIV-infected patients without malaria infection. Malaria was treated with artemether-lumefantrine (Coartem®). QFT testing was performed before initiation of malaria treatment and at days 7 and 42. In total, 172 patients completed follow-up. IFN-γ and IP-10 was measured in QFT supernatants. We found that during malaria infection IFN-γ and IP-10 levels in the unstimulated samples were elevated, mitogen responsiveness was impaired, and CD4 cell counts were decreased. These alterations reverted after malaria treatment. Concurrent malaria infection did not affect QFT test results, whereas there were more indeterminate IP-10 results during acute malaria infection. We suggest that IGRA and IP-10 release assay results of malaria patients should be interpreted with caution and that testing preferably should be postponed until after malaria treatment.
© The American Society of Tropical Medicine and Hygiene.
Conflict of interest statement
Disclosure: PR and MR hold a pending patent on the IP-10 release assay. MR is employed at Statens Serum Institut who holds and licenses IP on immunodiagnostic antigens included in QuantiFERON®. No other conflicts of interests declared.
Figures

Similar articles
-
Interferon-gamma inducible protein 10 as a biomarker for active tuberculosis and latent tuberculosis infection in children: a case-control study.Scand J Infect Dis. 2012 Apr;44(4):256-62. doi: 10.3109/00365548.2011.632644. Epub 2011 Nov 21. Scand J Infect Dis. 2012. PMID: 22103555
-
Thermostability of IFN-γ and IP-10 release assays for latent infection with Mycobacterium tuberculosis: A TBnet study.Tuberculosis (Edinb). 2016 May;98:7-12. doi: 10.1016/j.tube.2015.04.013. Epub 2015 May 15. Tuberculosis (Edinb). 2016. PMID: 27156612
-
A comparison of interferon-γ and IP-10 for the diagnosis of tuberculosis.Pediatrics. 2014 Dec;134(6):e1568-75. doi: 10.1542/peds.2014-1570. Pediatrics. 2014. PMID: 25422019
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Accuracy of interferon-γ-induced protein 10 for diagnosing latent tuberculosis infection: a systematic review and meta-analysis.Clin Microbiol Infect. 2019 Jun;25(6):667-672. doi: 10.1016/j.cmi.2018.12.006. Epub 2018 Dec 14. Clin Microbiol Infect. 2019. PMID: 30553864
Cited by
-
Malaria and tuberculosis co-infection-a review.Oxf Open Immunol. 2023 Nov 15;4(1):iqad008. doi: 10.1093/oxfimm/iqad008. eCollection 2023. Oxf Open Immunol. 2023. PMID: 38089636 Free PMC article. Review.
-
Distinct cytokine profiles in malaria coinfections: A systematic review.PLoS Negl Trop Dis. 2023 Jan 30;17(1):e0011061. doi: 10.1371/journal.pntd.0011061. eCollection 2023 Jan. PLoS Negl Trop Dis. 2023. PMID: 36716305 Free PMC article.
-
Examining the Complex Relationship Between Tuberculosis and Other Infectious Diseases in Children.Front Pediatr. 2019 Jun 25;7:233. doi: 10.3389/fped.2019.00233. eCollection 2019. Front Pediatr. 2019. PMID: 31294001 Free PMC article. Review.
-
Highly Multiplexed Proteomic Analysis of Quantiferon Supernatants To Identify Biomarkers of Latent Tuberculosis Infection.J Clin Microbiol. 2017 Feb;55(2):391-402. doi: 10.1128/JCM.01646-16. Epub 2016 Nov 16. J Clin Microbiol. 2017. PMID: 27852671 Free PMC article.
-
Positive Correlation between IP-10 and IFN-γ Levels in Rhesus Monkeys (Macaca mulatta) with Either Naturally Acquired or Experimental Infection of Mycobacterium tuberculosis.Biomed Res Int. 2017;2017:5089752. doi: 10.1155/2017/5089752. Epub 2017 Apr 20. Biomed Res Int. 2017. PMID: 28512637 Free PMC article.
References
-
- Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, Domínguez J, Duarte R, Ernst M, Eyuboglu FO, Gerogianni I, Girardi E, Goletti D, Janssens J-P, Julander I, Lange B, Latorre I, Losi M, Markova R, Matteelli A, Milburn H, Ravn P, Scholman T, Soccal PM, Straub M, Wagner D, Wolf T, Yalcin A, Lange C. TBNET Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 2014;190:1168–1176. - PubMed
-
- Vanini V, Petruccioli E, Gioia C, Cuzzi G, Orchi N, Rianda A, Alba L, Giancola ML, Conte A, Schininà V, Busi Rizzi E, Girardi E, Goletti D. IP-10 is an additional marker for tuberculosis (TB) detection in HIV-infected persons in a low-TB endemic country. J Infect. 2012;65:49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

