Hypersensitivity reactions associated with L-asparaginase administration in 142 dogs and 68 cats with lymphoid malignancies: 2007-2012
- PMID: 26834270
- PMCID: PMC4712998
Hypersensitivity reactions associated with L-asparaginase administration in 142 dogs and 68 cats with lymphoid malignancies: 2007-2012
Abstract
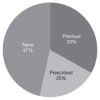
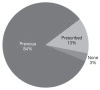
Clinically significant hypersensitivity reactions (HSRs) to the chemotherapy drug L-asparaginase are reported in humans and dogs, but frequency in small animals is not well-defined. This study retrospectively evaluated the frequency of HSR to L-asparaginase given by IM injection to dogs and cats with lymphoid malignancies. The medical records of all dogs and cats treated with at least 1 dose of L-asparaginase chemotherapy over a 5-year period were reviewed. A total of 370 doses of L-asparaginase were administered to the dogs, with 88 of 142 dogs receiving multiple doses, and 6 dogs experiencing an HSR. A total of 197 doses were administered to the cats, with 33 of 68 cats receiving multiple doses, and no cats experiencing an HSR. Hypersensitivity reactions were documented in 4.2% of dogs, and in association with 1.6% of L-asparaginase doses administered. These results show that HSRs occur uncommonly among dogs and cats, even with repeated dosing.
Réactions d’hypersensibilité associées à l’administration de L-asparaginase chez 142 chiens et 68 chats atteints de tumeurs malignes lymphoïdes: 2007–2012. Des réactions d’hypersensibilité cliniquement significatives (HCS) au médicament de chimiothérapie L-asparaginase sont signalées chez les humains et les chiens, mais leur fréquence chez les petits animaux n’est pas bien définie. Cette étude a évalué rétrospectivement la fréquence des HCS au médicament L-asparaginase administré par injection IM aux chiens et aux chats atteints de tumeurs malignes lymphoïdes. On a examiné les dossiers médicaux de tous les chiens et chats traités avec au moins une dose de chimiothérapie au médicament L-asparaginase pendant une période de 5 ans. Un total de 370 doses de L-asparaginase a été administré aux chiens, 88 des 142 chiens ont reçu des doses multiples et 6 chiens ont manifesté des HCS. Un total de 197 doses ont été administrées aux chats, 33 des 68 chats ont reçu des doses multiples et aucun chat n’a manifesté des HCS. Les HCS ont été documentées chez 4,2 % des chiens et en association avec 1,6 % des doses de L-asparaginase administrées. Ces résultats indiquent que les HCS se produisent rarement chez les chiens et les chats, même avec des doses répétées.(Traduit par Isabelle Vallières).
Figures
References
-
- Avramis VI, Panosyan EH. Pharmocokinectic/pharmacodynamic relationships of asparaginase formulations. Clin Pharmacokinet. 2005;44:367–393. - PubMed
-
- Chabner BA, Sallan SE. L-asparaginase. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2006. pp. 476–483.
-
- Raetz EA, Salzer WL. Tolerabilty and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–563. - PubMed
-
- Matus R, Leifer C, MacEwen EG. Acute lymphoblastic leukemia in the dog: A review of 30 cases. J Am Vet Med Assoc. 1983;183:859–862. - PubMed
-
- Matus RE. Chemotherapy of lymphoma and leukemia. In: Kirk RW, Bonagura JD, editors. Kirk’s Current Veterinary Therapy X. Philadelphia, Pennsylvania: WB Saunders; 1989. pp. 482–488.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
