A retrospective study of the real-life utilization and effectiveness of ranibizumab therapy for neovascular age-related macular degeneration in the UK
- PMID: 26834453
- PMCID: PMC4716754
- DOI: 10.2147/OPTH.S92627
A retrospective study of the real-life utilization and effectiveness of ranibizumab therapy for neovascular age-related macular degeneration in the UK
Abstract
Purpose: AURA was an international, retrospective, observational study that monitored the real-life use and effectiveness of ranibizumab injections in patients with neovascular age-related macular degeneration (nAMD). This paper reports the findings from the UK.
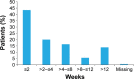
Methods: Patients who started treatment with ranibizumab between January 1, 2009, and August 31, 2009, and had documented follow-up to the end of their treatment and/or monitoring or until August 31, 2011, were retrospectively monitored; the diagnosis and subsequent decision to treat was made by the patient's own physician. Assessments included the change in visual acuity (standardized letter count) during the first and second years after start of ranibizumab therapy and resource utilization.
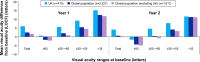
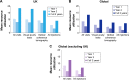
Results: Four hundred and ten patients from 13 UK centers were analyzed. The mean (standard deviation [SD]) letter score at baseline was 55.0 (17.8). The mean (SD) change in visual acuity from baseline was +6.0 (15.4) letters at year 1 and +4.1 (16.9) at year 2. Most of the patients (86.6%) completed a 3-month loading phase; the visual improvements were numerically higher in these patients. Over 2 years, the mean (SD) number of clinic visits and injections was 18.4 (5.0) and 9.0 (4.7), respectively. Resource use and visual acuity gains were greater than those observed in the global population, which included other countries enrolled in AURA (Canada, France, Germany, Ireland, Italy, the Netherlands, and Venezuela). When patients were stratified according to severity of nAMD (based on letter count at baseline), the mean change in visual acuity score at years 1 and 2 was also higher for the UK than for the global population across all subgroups.
Conclusion: Monitoring and treatment rates were high in the UK, resulting in better visual acuity outcomes compared with other included countries. This suggests that translation of clinical study outcomes into real-life settings is achievable, but at the expense of higher resource utilization than is currently the norm in most developed countries.
Keywords: anti-vascular endothelial growth factor; neovascular or wet age-related macular degeneration.
Figures

References
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Sadda SR, Stoller G, Boyer DS, Blodi BA, Shapiro H, Ianchulev T. Anatomical benefit from ranibizumab treatment of predominantly classic neovascular age-related macular degeneration in the 2-year anchor study. Retina. 2010;30(9):1390–1399. - PubMed
-
- Lucentis® (ranibizumab injection) [prescribing information] San Francisco, CA, USA: Genentech, Inc; 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

