Role of Glycogenolysis in Memory and Learning: Regulation by Noradrenaline, Serotonin and ATP
- PMID: 26834586
- PMCID: PMC4717441
- DOI: 10.3389/fnint.2015.00070
Role of Glycogenolysis in Memory and Learning: Regulation by Noradrenaline, Serotonin and ATP
Abstract
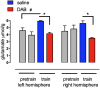
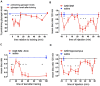
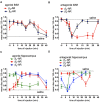
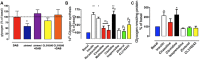
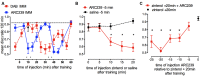
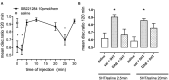
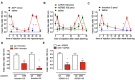
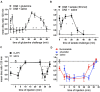
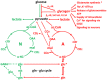
This paper reviews the role played by glycogen breakdown (glycogenolysis) and glycogen re-synthesis in memory processing in two different chick brain regions, (1) the hippocampus and (2) the avian equivalent of the mammalian cortex, the intermediate medial mesopallium (IMM). Memory processing is regulated by the neuromodulators noradrenaline and serotonin soon after training glycogen breakdown and re-synthesis. In day-old domestic chicks, memory formation is dependent on the breakdown of glycogen (glycogenolysis) at three specific times during the first 60 min after learning (around 2.5, 30, and 55 min). The chicks learn to discriminate in a single trial between beads of two colors and tastes. Inhibition of glycogen breakdown by the inhibitor of glycogen phosphorylase 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) given at specific times prior to the formation of long-term memory prevents memory forming. Noradrenergic stimulation of cultured chicken astrocytes by a selective β2-adrenergic (AR) agonist reduces glycogen levels and we believe that in vivo this triggers memory consolidation at the second stage of glycogenolysis. Serotonin acting at 5-HT2B receptors acts on the first stage, but not on the second. We have shown that noradrenaline, acting via post-synaptic α2-ARs, is also responsible for the synthesis of glycogen and our experiments suggest that there is a readily accessible labile pool of glycogen in astrocytes which is depleted within 10 min if glycogen synthesis is inhibited. Endogenous ATP promotion of memory consolidation at 2.5 and 30 min is also dependent on glycogen breakdown. ATP acts at P2Y1 receptors and the action of thrombin suggests that it causes the release of internal calcium ([Ca(2+)]i) in astrocytes. Glutamate and GABA, the primary neurotransmitters in the brain, cannot be synthesized in neurons de novo and neurons rely on astrocytic glutamate synthesis, requiring glycogenolysis.
Keywords: ATP; astrocytes; consolidation; day-old chickens; glycogen re-synthesis; memory processing; noradrenaline; serotonin.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

