The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury
- PMID: 26834638
- PMCID: PMC4712302
- DOI: 10.3389/fphar.2015.00326
The Extract of Roots of Sophora flavescens Enhances the Recovery of Motor Function by Axonal Growth in Mice with a Spinal Cord Injury
Abstract
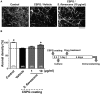
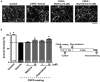
Although axonal extension to reconstruct spinal tracts should be effective for restoring function after spinal cord injury (SCI), chondroitin sulfate proteoglycan (CSPG) levels increase at spinal cord lesion sites, and inhibit axonal regrowth. In this study, we found that the water extract of roots of Sophora flavescens extended the axons of mouse cortical neurons, even on a CSPG-coated surface. Consecutive oral administrations of S. flavescens extract to SCI mice for 31 days increased the density of 5-HT-positive axons at the lesion site and improved the motor function. Further, the active constituents in the S. flavescens extract were identified. The water and alkaloid fractions of the S. flavescens extract each exhibited axonal extension activity in vitro. LC/MS analysis revealed that these fractions mainly contain matrine and/or oxymatrine, which are well-known major compounds in S. flavescens. Matrine and oxymatrine promoted axonal extension on the CSPG-coated surface. This study is the first to demonstrate that S. flavescens extract, matrine, and oxymatrine enhance axonal growth in vitro, even on a CSPG-coated surface, and that S. flavescens extract improves motor function and increases axonal density in SCI mice.
Keywords: Sophora flavescens; axonal growth; chondroitin sulfate proteoglycan; matrine; oxymatrine; spinal cord injury.
Figures





Similar articles
-
Matrine Directly Activates Extracellular Heat Shock Protein 90, Resulting in Axonal Growth and Functional Recovery in Spinal Cord Injured-Mice.Front Pharmacol. 2018 May 7;9:446. doi: 10.3389/fphar.2018.00446. eCollection 2018. Front Pharmacol. 2018. PMID: 29867458 Free PMC article.
-
Matrine promotes neural circuit remodeling to regulate motor function in a mouse model of chronic spinal cord injury.Neural Regen Res. 2019 Nov;14(11):1961-1967. doi: 10.4103/1673-5374.259625. Neural Regen Res. 2019. PMID: 31290454 Free PMC article.
-
Epac2 Elevation Reverses Inhibition by Chondroitin Sulfate Proteoglycans In Vitro and Transforms Postlesion Inhibitory Environment to Promote Axonal Outgrowth in an Ex Vivo Model of Spinal Cord Injury.J Neurosci. 2019 Oct 16;39(42):8330-8346. doi: 10.1523/JNEUROSCI.0374-19.2019. Epub 2019 Aug 13. J Neurosci. 2019. PMID: 31409666 Free PMC article.
-
[Development of New Therapies for Neurodegenerative Diseases via Axonal Growth].Yakugaku Zasshi. 2019;139(11):1385-1390. doi: 10.1248/yakushi.19-00147. Yakugaku Zasshi. 2019. PMID: 31685734 Review. Japanese.
-
Spinal cord regeneration.Cell Transplant. 2014;23(4-5):573-611. doi: 10.3727/096368914X678427. Cell Transplant. 2014. PMID: 24816452 Review.
Cited by
-
Matrine Directly Activates Extracellular Heat Shock Protein 90, Resulting in Axonal Growth and Functional Recovery in Spinal Cord Injured-Mice.Front Pharmacol. 2018 May 7;9:446. doi: 10.3389/fphar.2018.00446. eCollection 2018. Front Pharmacol. 2018. PMID: 29867458 Free PMC article.
-
The Synergistic Effect of Co-Treatment of Methyl Jasmonate and Cyclodextrins on Pterocarpan Production in Sophora flavescens Cell Cultures.Int J Mol Sci. 2020 May 30;21(11):3944. doi: 10.3390/ijms21113944. Int J Mol Sci. 2020. PMID: 32486319 Free PMC article.
-
Matrine Treatment Blocks NogoA-Induced Neural Inhibitory Signaling Pathway in Ongoing Experimental Autoimmune Encephalomyelitis.Mol Neurobiol. 2017 Dec;54(10):8404-8418. doi: 10.1007/s12035-016-0333-1. Epub 2016 Dec 9. Mol Neurobiol. 2017. PMID: 27933584
-
Pharmacological intervention for chronic phase of spinal cord injury.Neural Regen Res. 2025 May 1;20(5):1377-1389. doi: 10.4103/NRR.NRR-D-24-00176. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934397 Free PMC article.
-
Sedative Effect of Sophora flavescens and Matrine.Biomol Ther (Seoul). 2017 Jul 1;25(4):390-395. doi: 10.4062/biomolther.2016.156. Biomol Ther (Seoul). 2017. PMID: 28190318 Free PMC article.
References
-
- Bartus K., James N. D., Didangelos A., Bosch K. D., Verhaagen J., Yáñez-Muñoz R. J., et al. . (2014). Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. 34, 4822–4836. 10.1523/JNEUROSCI.4369-13.2014 - DOI - PMC - PubMed
-
- Commission of Chinese Pharmacopoeia (2010). Pharmacopoeia of the People's Republic of China, Vol. 1. Beijing: China Medical Science Press.
-
- Dev S., Mizuguchi H., Das A. K., Baba Y., Fukui H. (2011). Transcriptional microarray analysis reveals suppression of histamine signaling by Kujin alleviates allergic symptoms through down-regulation of FAT10 expression. Int. Immunopharmacol. 11, 1504–1509. 10.1016/j.intimp.2011.05.004 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

