Skeletal Muscle Mitochondrial Bioenergetics and Morphology in High Fat Diet Induced Obesity and Insulin Resistance: Focus on Dietary Fat Source
- PMID: 26834644
- PMCID: PMC4719079
- DOI: 10.3389/fphys.2015.00426
Skeletal Muscle Mitochondrial Bioenergetics and Morphology in High Fat Diet Induced Obesity and Insulin Resistance: Focus on Dietary Fat Source
Abstract
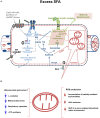
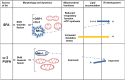
It has been suggested that skeletal muscle mitochondria play a key role in high fat (HF) diet induced insulin resistance (IR). Two opposite views are debated on mechanisms by which mitochondrial function could be involved in skeletal muscle IR. In one theory, mitochondrial dysfunction is suggested to cause intramyocellular lipid accumulation leading to IR. In the second theory, excess fuel within mitochondria in the absence of increased energy demand stimulates mitochondrial oxidant production and emission, ultimately leading to the development of IR. Noteworthy, mitochondrial bioenergetics is strictly associated with the maintenance of normal mitochondrial morphology by maintaining the balance between the fusion and fission processes. A shift toward mitochondrial fission with reduction of fusion protein, mainly mitofusin 2, has been associated with reduced insulin sensitivity and inflammation in obesity and IR development. However, dietary fat source during chronic overfeeding differently affects mitochondrial morphology. Saturated fatty acids induce skeletal muscle IR and inflammation associated with fission phenotype, whereas ω-3 polyunsaturated fatty acids improve skeletal muscle insulin sensitivity and inflammation, associated with a shift toward mitochondrial fusion phenotype. The present minireview focuses on mitochondrial bioenergetics and morphology in skeletal muscle IR, with particular attention to the effect of different dietary fat sources on skeletal muscle mitochondria morphology and fusion/fission balance.
Keywords: fish oil; lard; mitochondrial fission; mitochondrial fusion; omega-3 fatty acids.
Figures
Similar articles
-
Diet impact on mitochondrial bioenergetics and dynamics.Front Physiol. 2015 Apr 8;6:109. doi: 10.3389/fphys.2015.00109. eCollection 2015. Front Physiol. 2015. PMID: 25904870 Free PMC article. Review.
-
High-Fish Oil and High-Lard Diets Differently Affect Testicular Antioxidant Defense and Mitochondrial Fusion/Fission Balance in Male Wistar Rats: Potential Protective Effect of ω3 Polyunsaturated Fatty Acids Targeting Mitochondria Dynamics.Int J Mol Sci. 2019 Jun 25;20(12):3110. doi: 10.3390/ijms20123110. Int J Mol Sci. 2019. PMID: 31242698 Free PMC article.
-
Attenuation of obesity and insulin resistance by fish oil supplementation is associated with improved skeletal muscle mitochondrial function in mice fed a high-fat diet.J Nutr Biochem. 2018 May;55:76-88. doi: 10.1016/j.jnutbio.2017.11.012. Epub 2017 Dec 11. J Nutr Biochem. 2018. PMID: 29413492
-
High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria.PLoS One. 2014 Mar 24;9(3):e92753. doi: 10.1371/journal.pone.0092753. eCollection 2014. PLoS One. 2014. PMID: 24663492 Free PMC article.
-
Mitochondria-SR interaction and mitochondrial fusion/fission in the regulation of skeletal muscle metabolism.Metabolism. 2023 Jul;144:155578. doi: 10.1016/j.metabol.2023.155578. Epub 2023 May 8. Metabolism. 2023. PMID: 37164310 Review.
Cited by
-
Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2018 Sep;97(37):e12271. doi: 10.1097/MD.0000000000012271. Medicine (Baltimore). 2018. PMID: 30212963 Free PMC article.
-
Maternal Fat-1 Transgene Protects Offspring from Excess Weight Gain, Oxidative Stress, and Reduced Fatty Acid Oxidation in Response to High-Fat Diet.Nutrients. 2020 Mar 14;12(3):767. doi: 10.3390/nu12030767. Nutrients. 2020. PMID: 32183350 Free PMC article.
-
Dose- and Time-Dependent Effects of Oleate on Mitochondrial Fusion/Fission Proteins and Cell Viability in HepG2 Cells: Comparison with Palmitate Effects.Int J Mol Sci. 2021 Sep 10;22(18):9812. doi: 10.3390/ijms22189812. Int J Mol Sci. 2021. PMID: 34575980 Free PMC article.
-
Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes.Front Nutr. 2021 May 31;8:663838. doi: 10.3389/fnut.2021.663838. eCollection 2021. Front Nutr. 2021. PMID: 34136519 Free PMC article.
-
Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases.Int Rev Cell Mol Biol. 2018;340:209-344. doi: 10.1016/bs.ircmb.2018.05.006. Epub 2018 Jun 22. Int Rev Cell Mol Biol. 2018. PMID: 30072092 Free PMC article. Review.
References
-
- Bach D., Naon D., Pich S., Soriano F. X., Vega N., Rieusset J., et al. . (2005). Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 54, 2685–2693. 10.2337/diabetes.54.9.2685 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

