Oxytocin for Male Subjects with Autism Spectrum Disorder and Comorbid Intellectual Disabilities: A Randomized Pilot Study
- PMID: 26834651
- PMCID: PMC4720778
- DOI: 10.3389/fpsyt.2016.00002
Oxytocin for Male Subjects with Autism Spectrum Disorder and Comorbid Intellectual Disabilities: A Randomized Pilot Study
Abstract
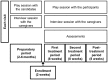
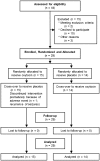
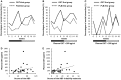
Approximately half of autism spectrum disorder (ASD) individuals suffer from comorbid intellectual disabilities (IDs). Oxytocin (OXT) receptors are highly expressed in temporal lobe structures and are likely to play a modulatory role in excitatory/inhibitory balance, at least based on animal model findings. Thus, it is feasible that in the highly representative group of Kanner-type ASD subjects, OXT could have a beneficial effect on social communication and social interaction. The aim of this pilot study is to investigate the feasibility and adverse events, such as epilepsy, of the long-term administration of intranasal OXT for adolescent and adult ASD subjects with ID because such patients frequently have seizures. We also addressed the question on how to scale the OXT effects to the core symptoms of social deficits because of the relative difficulty in obtaining objective measurements. Twenty-nine males (aged 15-40 years old) participated in a randomized, double-blind, and placebo-controlled crossover study (each for 8 weeks) with OXT (16 IU/day). Except for seizures experienced by one participant, other serious adverse events did not occur. The primary and secondary outcomes measured using the Childhood Autism Rating Scale and several standard scales, respectively, revealed no difference between the OXT and placebo groups. Instead, in an exploratory analysis, the social interactions observed in the play sessions or in daily life were significantly more frequent in the initial half period in the OXT-first arm of the crossover trial. There were also significant correlations between the plasma OXT concentration and subscale scores for irritability on the Aberrant Behavior Checklist. In conclusion, this pilot study demonstrates that long-term administration of intranasal OXT is tolerable in a representative cohort of ASD individuals with ID and suggests that future multicenter trials of OXT are warranted and should include measurements of reciprocal social interactions based on daily life under closer surveillance for epilepsy.
Trial registration: UMIN000007250.
Keywords: Kanner type; autism spectrum disorder; intranasal administration; oxytocin; social behavior.
Figures

References
-
- CDC: Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ (2012) 61:1–19. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed Washington, DC: American Psychiatric Association; (2013). p. 50–9.
-
- Kanner L. Autistic disturbance of affective contact. Nerv Child (1943) 2:217–50.
LinkOut - more resources
Full Text Sources
Other Literature Sources

