The Apoptotic Role of Metacaspase in Toxoplasma gondii
- PMID: 26834715
- PMCID: PMC4717298
- DOI: 10.3389/fmicb.2015.01560
The Apoptotic Role of Metacaspase in Toxoplasma gondii
Abstract
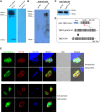
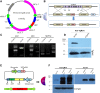
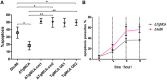
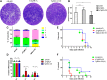
Toxoplasma gondii is a major opportunistic pathogen that spreads in a range of animal species and human beings. Quite a few characterizations of apoptosis have been identified in T. gondii treated with apoptosis inducers, but the molecular mechanisms of the pathway are not clearly understood. Metacaspases are caspase-like cysteine proteases that can be found in plants, fungi, and protozoa in which caspases are absent. Metacaspases are multifunctional proteases involved in apoptosis-like cell death, insoluble protein aggregate clearance, and cell proliferation. To investigate whether T. gondii metacaspase (TgMCA) is involved in the apoptosis of the parasites, we generated TgMCA mutant strains. Western blot analysis indicated that the autoproteolytic processing of TgMCA was the same as that for metacaspases of some other species. Indirect immunofluorescence assay (IFA) showed that TgMCA was dispersed throughout the cytoplasm and relocated to the nucleus when the parasites were exposed to the extracellular environment, which indicated the execution of its function in the nucleus. The number of apoptosis parasites was significantly diminished in the TgMCA knockout strain and increased in the TgMCA overexpression strain after treatment with extracellular buffer, as determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The lack of TgMCA did not affect the parasite propagation in vitro and virulence in vivo, suggesting that it is probably redundant in parasite propagation. But overexpression of TgMCA reduced the intracellular parasites growth in vitro. The TgMCA knockout strain showed more viability in extracellular buffer compared to the parental and overexpression lines. In this study, we demonstrated that TgMCA contributes to the apoptosis of T. gondii.
Keywords: T. gondii; apoptosis; knockout; metacaspase; programmed cell death.
Figures

Similar articles
-
Requirement of Toxoplasma gondii metacaspases for IMC1 maturation, endodyogeny and virulence in mice.Parasit Vectors. 2021 Aug 12;14(1):400. doi: 10.1186/s13071-021-04878-0. Parasit Vectors. 2021. PMID: 34384491 Free PMC article.
-
Apoptosis-like cell death pathways in the unicellular parasite Toxoplasma gondii following treatment with apoptosis inducers and chemotherapeutic agents: a proof-of-concept study.Apoptosis. 2013 Jun;18(6):664-80. doi: 10.1007/s10495-013-0832-8. Apoptosis. 2013. PMID: 23468121 Free PMC article.
-
[Metacaspases and their role in the life cycle of human protozoan parasites].Biomedica. 2009 Sep;29(3):485-93. Biomedica. 2009. PMID: 20436999 Spanish.
-
Regulating Death and Disease: Exploring the Roles of Metacaspases in Plants and Fungi.Int J Mol Sci. 2022 Dec 24;24(1):312. doi: 10.3390/ijms24010312. Int J Mol Sci. 2022. PMID: 36613753 Free PMC article. Review.
-
Caspases in plants: metacaspase gene family in plant stress responses.Funct Integr Genomics. 2015 Nov;15(6):639-49. doi: 10.1007/s10142-015-0459-7. Epub 2015 Aug 16. Funct Integr Genomics. 2015. PMID: 26277721 Review.
Cited by
-
Identification and function characterization of NcAP2XII-4 in Neospora caninum.Parasit Vectors. 2024 Sep 14;17(1):392. doi: 10.1186/s13071-024-06477-1. Parasit Vectors. 2024. PMID: 39277758 Free PMC article.
-
A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii.PLoS Pathog. 2022 Jul 5;18(7):e1010665. doi: 10.1371/journal.ppat.1010665. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35788770 Free PMC article.
-
Immunization with the NcMYR1 gene knockout strain effectively protected C57BL/6 mice and their pups against the Neospora caninum challenge.Virulence. 2024 Dec;15(1):2427844. doi: 10.1080/21505594.2024.2427844. Epub 2024 Nov 28. Virulence. 2024. PMID: 39607301 Free PMC article.
-
Development and validation of a machine learning algorithm prediction for dense granule proteins in Apicomplexa.Parasit Vectors. 2023 Mar 14;16(1):98. doi: 10.1186/s13071-023-05698-0. Parasit Vectors. 2023. PMID: 36918932 Free PMC article.
-
Stable Transfection of Eimeria intestinalis and Investigation of Its Life Cycle, Reproduction and Immunogenicity.Front Microbiol. 2016 May 31;7:807. doi: 10.3389/fmicb.2016.00807. eCollection 2016. Front Microbiol. 2016. PMID: 27303389 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

