Biosurfactant Produced by Salmonella Enteritidis SE86 Can Increase Adherence and Resistance to Sanitizers on Lettuce Leaves (Lactuca sativa L., cichoraceae)
- PMID: 26834727
- PMCID: PMC4722381
- DOI: 10.3389/fmicb.2016.00009
Biosurfactant Produced by Salmonella Enteritidis SE86 Can Increase Adherence and Resistance to Sanitizers on Lettuce Leaves (Lactuca sativa L., cichoraceae)
Abstract
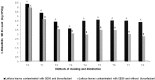
Salmonella Enteritidis SE86 is an important foodborne pathogen in Southern Brazil and it is able to produce a biosurfactant. However, the importance of this compound for the microorganism is still unknown. This study aimed to investigate the influence of the biosurfactant produced by S. Enteritidis SE86 on adherence to slices of lettuce leaves and on resistance to sanitizers. First, lettuce leaves were inoculated with S. Enteritidis SE86 in order to determine the amount of biosurfactant produced. Subsequently, lettuce leaves were inoculated with S. Enteritidis SE86 with and without the biosurfactant, and the adherence and bacterial resistance to different sanitization methods were evaluated. S. Enteritidis SE86 produced biosurfactant after 16 h (emulsification index of 11 to 52.15 percent, P < 0.05) and showed greater adherence capability and resistance to sanitization methods when the compound was present. The scanning electron microscopy demonstrated that S. Enteritidis was able to adhere, form lumps, and invade the lettuce leaves' stomata in the presence of the biosurfactant. Results indicated that the biosurfactant produced by S. Enteritidis SE86 contributed to adherence and increased resistance to sanitizers when the microorganism was present on lettuce leaves.
Keywords: Salmonella Enteritidis SE86; biosurfactant; disinfection; lettuce; microbial adherence and resistance.
Figures


Similar articles
-
Investigation of rpoS and dps genes in sodium hypochlorite resistance of Salmonella Enteritidis SE86 isolated from foodborne illness outbreaks in southern Brazil.J Food Prot. 2012 Mar;75(3):437-42. doi: 10.4315/0362-028X.JFP-11-286. J Food Prot. 2012. PMID: 22410215
-
Expression of ompR gene in the acid adaptation and thermal resistance of Salmonella Enteritidis SE86.J Infect Dev Ctries. 2014 Apr 15;8(4):474-9. doi: 10.3855/jidc.3584. J Infect Dev Ctries. 2014. PMID: 24727514
-
Antagonistic activity of Lactobacillus acidophilus LA10 against Salmonella enterica serovar Enteritidis SE86 in mice.Braz J Microbiol. 2013 May 7;44(1):57-61. doi: 10.1590/S1517-83822013005000024. eCollection 2013. Braz J Microbiol. 2013. PMID: 24159284 Free PMC article.
-
Phenotypic and Molecular Characterization of Salmonella Enteritidis SE86 Isolated from Poultry and Salmonellosis Outbreaks.Foodborne Pathog Dis. 2017 Dec;14(12):742-754. doi: 10.1089/fpd.2017.2327. Epub 2017 Nov 6. Foodborne Pathog Dis. 2017. PMID: 29106298
-
Modeling Growth Kinetic Parameters of Salmonella Enteritidis SE86 on Homemade Mayonnaise Under Isothermal and Nonisothermal Conditions.Foodborne Pathog Dis. 2016 Aug;13(8):462-7. doi: 10.1089/fpd.2015.2045. Epub 2016 Feb 9. Foodborne Pathog Dis. 2016. PMID: 26859536
Cited by
-
Exopolymeric substances (EPS) from Salmonella enterica: polymers, proteins and their interactions with plants and abiotic surfaces.J Microbiol. 2019 Jan;57(1):1-8. doi: 10.1007/s12275-019-8353-y. Epub 2018 Sep 6. J Microbiol. 2019. PMID: 30552630 Review.
-
Invasion of Epithelial Cells Is Correlated with Secretion of Biosurfactant via the Type 3 Secretion System (T3SS) of Shigella flexneri.J Pathog. 2020 Jul 27;2020:3062821. doi: 10.1155/2020/3062821. eCollection 2020. J Pathog. 2020. PMID: 32802515 Free PMC article.
References
-
- Abreu I. M. O., Junqueira A. M. R., Peixoto J. R., Oliveira S. A. (2010). Qualidade microbiológica e produtividade de alface sob adubação química e orgânica. Ciênc. Tecnol. Alimentos 30 108–118. 10.1590/S0101-20612010000500018 - DOI
-
- An Y. H., Skowronski P. (2000). “General considerations for studying bacterial adhesion to biomaterials,” in Handbook of Bacterial Adhesion: Principles, Methods and Applications, eds An H. H., Friedman R. J. (Berlin: Springer Science and Business Media; ), 121–132.
-
- Antunes M. A. (2009). Contaminação Crescimento e Inativação de Microrganismos na Cadeia de Produção da Alface (Lactuca sativa) Propriedade de Santo Antão. Ph.D. thesis, Universidade Federal de Viçosa, Minas Gerais.
LinkOut - more resources
Full Text Sources
Other Literature Sources

