Role of HLA Adaptation in HIV Evolution
- PMID: 26834742
- PMCID: PMC4716577
- DOI: 10.3389/fimmu.2015.00665
Role of HLA Adaptation in HIV Evolution
Abstract
Killing of HIV-infected cells by CD8(+) T-cells imposes strong selection pressure on the virus toward escape. The HLA class I molecules that are successful in mediating some degree of control over the virus are those that tend to present epitopes in conserved regions of the proteome, such as in p24 Gag, in which escape also comes at a significant cost to viral replicative capacity (VRC). In some instances, compensatory mutations can fully correct for the fitness cost of such an escape variant; in others, correction is only partial. The consequences of these events within the HIV-infected host, and at the population level following transmission of escape variants, are discussed. The accumulation of escape mutants in populations over the course of the epidemic already shows instances of protective HLA molecules losing their impact, and in certain cases, a modest decline in HIV virulence in association with population-level increase in mutants that reduce VRC.
Keywords: CD8+ T cells; HIV-1; HLA class I; viral adaptation; viral fitness; viral replicative capacity.
Figures

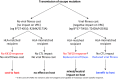
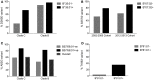
References
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet (2008) 372:1881–93.10.1016/S0140-6736(08)61591-3 - DOI - PMC - PubMed
-
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis (2011) 11:507–15.10.1016/S1473-3099(11)70098-6 - DOI - PMC - PubMed
-
- Gray GE, Moodie Z, Metch B, Gilbert PB, Bekker LG, Churchyard G, et al. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Lancet Infect Dis (2014) 14:388–96.10.1016/S1473-3099(14)70020-9 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

