Tolerogenic IDO(+) Dendritic Cells Are Induced by PD-1-Expressing Mast Cells
- PMID: 26834749
- PMCID: PMC4724729
- DOI: 10.3389/fimmu.2016.00009
Tolerogenic IDO(+) Dendritic Cells Are Induced by PD-1-Expressing Mast Cells
Abstract
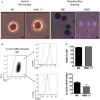
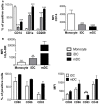
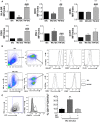
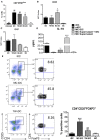
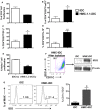
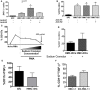
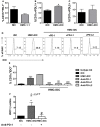
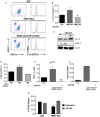
Mast cells (MCs) are tissue resident cells, rich in inflammatory mediators, involved in allergic reactions, and with an increasingly recognized role in immunomodulation. Dendritic cells (DCs), on the other hand, are central to the determination of immune response patterns, being highly efficient antigen-presenting cells that respond promptly to changes in their microenvironment. Here, we show that direct cell contact between immature monocyte-derived DCs (iDCs) and MC bends DCs toward tolerance induction. DCs that had direct contact with MC (MC-iDC) decreased HLA-DR but increased PD-L1 expression and stimulated regulatory T lymphocytes, which expresses FoxP3(+), secrete TGF-β and IL-10, and suppress the proliferation of mitogen-stimulated naïve T lymphocytes. Furthermore, MC-iDC expressed higher levels of indoleamine-2,3-deoxigenase (IDO), a phenomenon that was blocked by treatment of MC with anti-PD-1 or by the treatment of DCs with anti-PD-L1 or anti-PD-L2, but not by blocking of H1 and H2 histamine receptors on DCs. Contact with MC also increased phosphorylated STAT-3 levels in iDCs. When a STAT-3 inhibitor, JSI-124, was added to the DCs before contact with MC, the MC-iDC recovered their ability to induce allogeneic T cell proliferation and did not increase their IDO expression.
Keywords: IDO; STAT-3; Tregs; mast cells; tolerogenic DCs.
Figures

Similar articles
-
Modulatory effect of rapamycin and tacrolimus on monocyte-derived dendritic cells phenotype and function.Immunobiology. 2021 Jan;226(1):152031. doi: 10.1016/j.imbio.2020.152031. Epub 2020 Nov 23. Immunobiology. 2021. PMID: 33278711
-
Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics.J Cell Biochem. 2018 Sep;119(9):7865-7872. doi: 10.1002/jcb.27203. Epub 2018 Jun 26. J Cell Biochem. 2018. PMID: 29943843
-
Trichinella spiralis Excretory-Secretory Products Induce Tolerogenic Properties in Human Dendritic Cells via Toll-Like Receptors 2 and 4.Front Immunol. 2018 Jan 24;9:11. doi: 10.3389/fimmu.2018.00011. eCollection 2018. Front Immunol. 2018. PMID: 29416536 Free PMC article.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
Cited by
-
PD-1+ mast cell enhanced by PD-1 blocking therapy associated with resistance to immunotherapy.Cancer Immunol Immunother. 2023 Mar;72(3):633-645. doi: 10.1007/s00262-022-03282-6. Epub 2022 Aug 26. Cancer Immunol Immunother. 2023. PMID: 36018370 Free PMC article.
-
Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives.Front Immunol. 2022 Jan 31;13:807271. doi: 10.3389/fimmu.2022.807271. eCollection 2022. Front Immunol. 2022. PMID: 35173722 Free PMC article. Review.
-
Aryl hydrocarbon receptor activation ameliorates experimental colitis by modulating the tolerogenic dendritic and regulatory T cell formation.Cell Biosci. 2022 Apr 23;12(1):46. doi: 10.1186/s13578-022-00780-z. Cell Biosci. 2022. PMID: 35461286 Free PMC article.
-
Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations.Front Oncol. 2018 Mar 28;8:86. doi: 10.3389/fonc.2018.00086. eCollection 2018. Front Oncol. 2018. PMID: 29644214 Free PMC article. Review.
-
Longitudinal cytokine and multi-modal health data of an extremely severe ME/CFS patient with HSD reveals insights into immunopathology, and disease severity.Front Immunol. 2024 Apr 8;15:1369295. doi: 10.3389/fimmu.2024.1369295. eCollection 2024. Front Immunol. 2024. PMID: 38650940 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

