Nitric Oxide-Mediated Maize Root Apex Responses to Nitrate are Regulated by Auxin and Strigolactones
- PMID: 26834770
- PMCID: PMC4722128
- DOI: 10.3389/fpls.2015.01269
Nitric Oxide-Mediated Maize Root Apex Responses to Nitrate are Regulated by Auxin and Strigolactones
Abstract
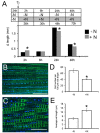
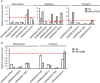
Nitrate (NO3 (-)) is a key element for crop production but its levels in agricultural soils are limited. Plants have developed mechanisms to cope with these NO3 (-) fluctuations based on sensing nitrate at the root apex. Particularly, the transition zone (TZ) of root apex has been suggested as a signaling-response zone. This study dissects cellular and molecular mechanisms underlying NO3 (-) resupply effects on primary root (PR) growth in maize, confirming nitric oxide (NO) as a putative modulator. Nitrate restoration induced PR elongation within the first 2 h, corresponding to a stimulation of cell elongation at the basal border of the TZ. Xyloglucans (XGs) immunolocalization together with Brefeldin A applications demonstrated that nitrate resupply induces XG accumulation. This effect was blocked by cPTIO (NO scavenger). Transcriptional analysis of ZmXET1 confirmed the stimulatory effect of nitrate on XGs accumulation in cells of the TZ. Immunolocalization analyses revealed a positive effect of nitrate resupply on auxin and PIN1 accumulation, but a transcriptional regulation of auxin biosynthesis/transport/signaling genes was excluded. Short-term nitrate treatment repressed the transcription of genes involved in strigolactones (SLs) biosynthesis and transport, mainly in the TZ. Enhancement of carotenoid cleavage dioxygenases (CCDs) transcription in presence of cPTIO indicated endogenous NO as a negative modulator of CCDs activity. Finally, treatment with the SLs-biosynthesis inhibitor (TIS108) restored the root growth in the nitrate-starved seedlings. Present report suggests that the NO-mediated root apex responses to nitrate are accomplished in cells of the TZ via integrative actions of auxin, NO and SLs.
Keywords: auxin; nitrate; nitric oxide; root; strigolactones; transition zone.
Figures








Similar articles
-
Local regulation of auxin transport in root-apex transition zone mediates aluminium-induced Arabidopsis root-growth inhibition.Plant J. 2021 Oct;108(1):55-66. doi: 10.1111/tpj.15424. Epub 2021 Jul 30. Plant J. 2021. PMID: 34273207
-
Nitrate sensing by the maize root apex transition zone: a merged transcriptomic and proteomic survey.J Exp Bot. 2015 Jul;66(13):3699-715. doi: 10.1093/jxb/erv165. Epub 2015 Apr 23. J Exp Bot. 2015. PMID: 25911739 Free PMC article.
-
Nitric oxide mediates strigolactone signaling in auxin and ethylene-sensitive lateral root formation in sunflower seedlings.Plant Signal Behav. 2015;10(8):e1054087. doi: 10.1080/15592324.2015.1054087. Plant Signal Behav. 2015. PMID: 26076049 Free PMC article.
-
New Paradigms in Brassinosteroids, Strigolactones, Sphingolipids, and Nitric Oxide Interaction in the Control of Lateral and Adventitious Root Formation.Plants (Basel). 2023 Jan 16;12(2):413. doi: 10.3390/plants12020413. Plants (Basel). 2023. PMID: 36679126 Free PMC article. Review.
-
Signalling Overlaps between Nitrate and Auxin in Regulation of The Root System Architecture: Insights from the Arabidopsis thaliana.Int J Mol Sci. 2020 Apr 20;21(8):2880. doi: 10.3390/ijms21082880. Int J Mol Sci. 2020. PMID: 32326090 Free PMC article. Review.
Cited by
-
Physiological and Nutritional Responses of Pear Seedlings to Nitrate Concentrations.Front Plant Sci. 2018 Nov 20;9:1679. doi: 10.3389/fpls.2018.01679. eCollection 2018. Front Plant Sci. 2018. PMID: 30515181 Free PMC article.
-
Interaction between fructan metabolism and plant growth regulators.Planta. 2022 Jan 27;255(2):49. doi: 10.1007/s00425-022-03826-1. Planta. 2022. PMID: 35084581 Review.
-
The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants.Planta. 2020 Mar 18;251(4):84. doi: 10.1007/s00425-020-03376-4. Planta. 2020. PMID: 32189077 Review.
-
Nitric Oxide Affects Rice Root Growth by Regulating Auxin Transport Under Nitrate Supply.Front Plant Sci. 2018 May 23;9:659. doi: 10.3389/fpls.2018.00659. eCollection 2018. Front Plant Sci. 2018. PMID: 29875779 Free PMC article.
-
Effects of NH4 +-N: NO3 --N ratio on growth, nutrient uptake and production of blueberry (Vaccinium spp.) under soilless culture.Front Plant Sci. 2024 Oct 17;15:1438811. doi: 10.3389/fpls.2024.1438811. eCollection 2024. Front Plant Sci. 2024. PMID: 39502920 Free PMC article.
References
-
- Andrews M., Raven J. A., Lea P. J. (2013). Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 163 174–199. 10.1111/aab.12045 - DOI
-
- Baligar V. C., Fageria N. K., He Z. L. (2001). Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 32 921–950. 10.1081/CSS-100104098 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

