MinION Analysis and Reference Consortium: Phase 1 data release and analysis
- PMID: 26834992
- PMCID: PMC4722697
- DOI: 10.12688/f1000research.7201.1
MinION Analysis and Reference Consortium: Phase 1 data release and analysis
Abstract
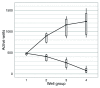
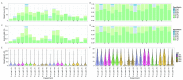
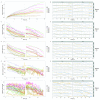
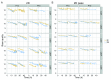
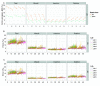
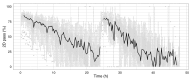
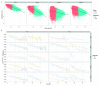
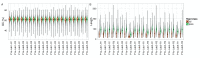
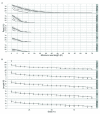
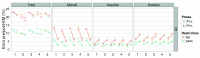
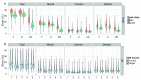
The advent of a miniaturized DNA sequencing device with a high-throughput contextual sequencing capability embodies the next generation of large scale sequencing tools. The MinION™ Access Programme (MAP) was initiated by Oxford Nanopore Technologies™ in April 2014, giving public access to their USB-attached miniature sequencing device. The MinION Analysis and Reference Consortium (MARC) was formed by a subset of MAP participants, with the aim of evaluating and providing standard protocols and reference data to the community. Envisaged as a multi-phased project, this study provides the global community with the Phase 1 data from MARC, where the reproducibility of the performance of the MinION was evaluated at multiple sites. Five laboratories on two continents generated data using a control strain of Escherichia coli K-12, preparing and sequencing samples according to a revised ONT protocol. Here, we provide the details of the protocol used, along with a preliminary analysis of the characteristics of typical runs including the consistency, rate, volume and quality of data produced. Further analysis of the Phase 1 data presented here, and additional experiments in Phase 2 of E. coli from MARC are already underway to identify ways to improve and enhance MinION performance.
Keywords: MinION; NanoOK; data release; long reads; marginAlign; minoTour; nanopore sequencing; third-generation sequencing.
Conflict of interest statement
Figures










References
-
- Check Hayden E: Nanopore genome sequencer makes its debut. Nat News. 2012. 10.1038/nature.2012.10051 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

