A multicentre, randomised controlled study of enteric-coated mycophenolate sodium for the treatment of relapsed or resistant proliferative lupus nephritis: an Asian experience
- PMID: 26835147
- PMCID: PMC4716419
- DOI: 10.1136/lupus-2015-000120
A multicentre, randomised controlled study of enteric-coated mycophenolate sodium for the treatment of relapsed or resistant proliferative lupus nephritis: an Asian experience
Abstract
Objective: The optimal treatment of relapse or resistant lupus nephritis (LN) is still unclear. Mycophenolate might be an alternative therapy to avoid toxicities of cyclophosphamide (CYC). This study was aimed to compare enteric-coated mycophenolate sodium (EC-MPS) versus intravenous CYC as an induction therapy.
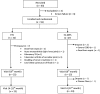
Methods: The study was a 12-month period of multicentre, open-labelled randomised controlled trial. Fifty-nine patients who had relapsed (36%) or who were resistant to previous CYC treatment (64%) and all who were biopsy-proven class III/IV, were randomised into CYC (n=32) and EC-MPS groups (n=27). The CYC group received intravenous CYC 0.5-1 g/m(2) monthly and the EC-MPS group was treated with EC-MPS 1440 mg/day for first 6 months. After induction therapy, both groups received EC-MPS 720 mg/day until the end of study at 12 months.
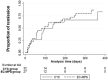
Results: The study was prematurely terminated due to high rate of serious adverse events in CYC arm. Death and serious infections were observed more in the CYC group (15.6% in CYC and 3.5% in EC-MPS; p=0.04). The early discontinuation rates, mainly from serious infections, were significantly higher in CYC group (percentage differences of 16.9; 95% CI 1.3 to 32.4). At the 12th month, both arms were comparable in terms of complete and partial remission rates (68% CYC and 71% EC-MPS) and times to remission (96 days CYC and 97 days EC-MPS). Composites of unfavourable outcomes (death, doubling of serum creatinine, non-remission and intolerance to treatment) were 46.9% and 37% in CYC and EC-MPS (risk difference=9.84; p=0.44).
Conclusions: EC-MPS may have comparable efficacy, but was better tolerated than CYC. EC-MPS should be an alternative choice of treatment for difficult-to-treat LN, particularly in CYC-experienced LN patients. Due to an early termination of the study, further clinical implementation could be cautiously used.
Trial registration number: Clinicaltrials.gov ID#NCT01015456.
Keywords: Cyclophosphamide; Infections; Lupus Nephritis; Treatment.
Figures

References
-
- Ortega LM, Schultz DR, Lenz O et al. Lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 2010;19:557–74. doi:10.1177/0961203309358187 - DOI - PubMed
-
- Gamba G, Quintanilla L, del Bosque MD et al. Clinical course and prognostic factors in lupus nephropathy. Rev Invest Clin 2000;52:397–405. - PubMed
-
- Sprangers B, Monahan M, Appel GB. Diagnosis and treatment of lupus nephritis flares--an update. Nat Rev Nephrol 2012;8:709–17. doi:10.1038/nrneph.2012.220 - DOI - PubMed
-
- Mok CC, Yap DY, Navarra SV et al. Overview of lupus nephritis management guidelines and perspective from Asia. Nephrology (Carlton) 2014;19:11–20. doi:10.1111/nep.12136 - DOI - PubMed
-
- Jakes RW, Bae S-C, Louthrenoo W et al. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res 2012;64:159–68. doi:10.1002/acr.20683 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
