New building blocks or dendritic pseudopeptides for metal chelating
- PMID: 26835235
- PMCID: PMC4720618
- DOI: 10.1186/s40064-016-1703-x
New building blocks or dendritic pseudopeptides for metal chelating
Abstract
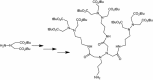
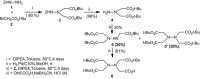
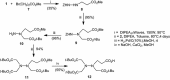
Dendritic oligopeptides have been reported as useful building blocks for many interactions. Starting from hydrazine, we described an approach to create new dendritic pseudopeptides linked with biological systems, such as cell membrane, as chelate metal, Ni(2+)-nitrilotriacetic acid moieties which could target histidine rich peptides or proteins. Depending on the nature of these new chemical recognition units, they could be integrated into a peptide by coupling in C or N-termini.Graphical abstract:Dendrimer formation.
Keywords: Aza-NTA; Aza-β3-amino acids; Aza-β3-peptides; Dendritic pseudopeptides.
Figures
Similar articles
-
Development and characterization of Ni-NTA-bearing microspheres.Cytometry. 2002 Jul 1;48(3):136-45. doi: 10.1002/cyto.10124. Cytometry. 2002. PMID: 12116359
-
De novo cyclic pseudopeptides containing aza-β3-amino acids exhibiting antimicrobial activities.J Med Chem. 2012 Dec 27;55(24):10885-95. doi: 10.1021/jm3009037. Epub 2012 Dec 6. J Med Chem. 2012. PMID: 23148564
-
Metal-chelating amino acids as building blocks for synthetic receptors sensing metal ions and histidine-tagged proteins.Chembiochem. 2003 Dec 5;4(12):1340-4. doi: 10.1002/cbic.200200455. Chembiochem. 2003. PMID: 14661277
-
Sugar amino acids and their uses in designing bioactive molecules.Curr Med Chem. 2002 Feb;9(4):421-35. doi: 10.2174/0929867023370941. Curr Med Chem. 2002. PMID: 11945118 Review.
-
A review of the environmental and mammalian toxicology of nitrilotriacetic acid.Crit Rev Toxicol. 1985;15(1):1-102. doi: 10.3109/10408448509023766. Crit Rev Toxicol. 1985. PMID: 3899518 Review.
Cited by
-
Biosensors Based on the Binding Events of Nitrilotriacetic Acid-Metal Complexes.Biosensors (Basel). 2023 Apr 28;13(5):507. doi: 10.3390/bios13050507. Biosensors (Basel). 2023. PMID: 37232868 Free PMC article. Review.
References
-
- Abbour S, Baudy-Floc’h M. Fmoc-aza-β3-lys-OAllyl and Fmoc-aza-β3-asp-OAllyl for on-resin head-to-tail cyclization of aza-β3-peptides. Tetrahedron Lett. 2013;54:775–778. doi: 10.1016/j.tetlet.2012.11.113. - DOI
-
- Busnel O, Baudy-Floc’h M. Preparation of Fmoc-Aza-β3-Pro-OH, Fmoc-Aza-β3-Asn-OH, Fmoc-Aza-β3-Asp-OH, Fmoc-Aza-β3-Glu-OH for solid-phase syntheses of Aza-β3-peptides. Tetrahedron Lett. 2007;48:5767–5770. doi: 10.1016/j.tetlet.2007.06.082. - DOI
-
- Gentilucci L, De Marco R, Cerisoli L (2010) Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Cur Pharm Des 16(28):3185–3203 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources