Past, present and future of host-parasite co-extinctions
- PMID: 26835251
- PMCID: PMC4699984
- DOI: 10.1016/j.ijppaw.2015.08.007
Past, present and future of host-parasite co-extinctions
Abstract
Human induced ecosystem alterations and climate change are expected to drive several species to extinction. In this context, the attention of public opinion, and hence conservationists' efforts, are often targeted towards species having emotional, recreational and/or economical value. This tendency may result in a high number of extinctions happening unnoticed. Among these, many could involve parasites. Several studies have highlighted various reasons why we should care about this, that go far beyond the fact that parasites are amazingly diverse. A growing corpus of evidence suggests that parasites contribute much to ecosystems both in terms of biomass and services, and the seemingly paradoxical idea that a healthy ecosystem is one rich in parasites is becoming key to the whole concept of parasite conservation. Although various articles have covered different aspects of host-parasite co-extinctions, I feel that some important conceptual issues still need to be formally addressed. In this review, I will attempt at clarifying some of them, with the aim of providing researchers with a unifying conceptual framework that could help them designing future studies. In doing this, I will try to draw a more clear distinction between the (co-)evolutionary and the ecological dimensions of co-extinction studies, since the ongoing processes that are putting parasites at risk now operate at a scale that is extremely different from the one that has shaped host-parasite networks throughout million years of co-evolution. Moreover, I will emphasize how the complexity of direct and indirect effects of parasites on ecosystems makes it much challenging to identify the mechanisms possibly leading to co-extinction events, and to predict how such events will affect ecosystems in the long run.
Keywords: Biological invasions; Climate change; Ecological networks; Enemy release; Host range; Host-specificity; IUCN; Indirect competition.
Figures
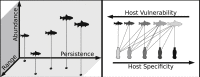

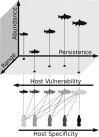
Similar articles
-
Predictions of primate-parasite coextinction.Philos Trans R Soc Lond B Biol Sci. 2021 Nov 8;376(1837):20200355. doi: 10.1098/rstb.2020.0355. Epub 2021 Sep 20. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 34538137 Free PMC article.
-
Estimating co-extinction threats in terrestrial ecosystems.Glob Chang Biol. 2023 Sep;29(18):5122-5138. doi: 10.1111/gcb.16836. Epub 2023 Jun 29. Glob Chang Biol. 2023. PMID: 37386726 Review.
-
Ecological and evolutionary legacy of megafauna extinctions.Biol Rev Camb Philos Soc. 2018 May;93(2):845-862. doi: 10.1111/brv.12374. Epub 2017 Oct 9. Biol Rev Camb Philos Soc. 2018. PMID: 28990321 Review.
-
Patterns of host-parasite coinvasion promote enemy release and specialist parasite spillover.J Anim Ecol. 2023 May;92(5):1029-1041. doi: 10.1111/1365-2656.13910. Epub 2023 Apr 4. J Anim Ecol. 2023. PMID: 36934311
-
A few good reasons why species-area relationships do not work for parasites.Biomed Res Int. 2014;2014:271680. doi: 10.1155/2014/271680. Epub 2014 May 8. Biomed Res Int. 2014. PMID: 24895561 Free PMC article. Review.
Cited by
-
Australian spiny mountain crayfish and their temnocephalan ectosymbionts: an ancient association on the edge of coextinction?Proc Biol Sci. 2016 May 25;283(1831):20160585. doi: 10.1098/rspb.2016.0585. Proc Biol Sci. 2016. PMID: 27226467 Free PMC article.
-
Mussel parasite richness and risk of extinction.Conserv Biol. 2022 Dec;36(6):e13979. doi: 10.1111/cobi.13979. Epub 2022 Sep 26. Conserv Biol. 2022. PMID: 35929586 Free PMC article.
-
The pervasive impact of global climate change on plant-nematode interaction continuum.Front Plant Sci. 2023 Apr 6;14:1143889. doi: 10.3389/fpls.2023.1143889. eCollection 2023. Front Plant Sci. 2023. PMID: 37089646 Free PMC article. Review.
-
High levels of inbreeding with spatial and host-associated structure in lice of an endangered freshwater seal.Mol Ecol. 2022 Sep;31(18):4593-4606. doi: 10.1111/mec.16569. Epub 2022 Jul 1. Mol Ecol. 2022. PMID: 35726520 Free PMC article.
-
Time-travelling pathogens and their risk to ecological communities.PLoS Comput Biol. 2023 Jul 27;19(7):e1011268. doi: 10.1371/journal.pcbi.1011268. eCollection 2023 Jul. PLoS Comput Biol. 2023. PMID: 37498846 Free PMC article.
References
-
- Adamson M.L., Caira J.N. Evolutionary factors influencing the nature of parasite specificity. Parasitology. 1994;109(S1):S85–S95. - PubMed
-
- Alonso P.L., Brown G., Arevalo-Herrera M., Binka F., Chitnis C., Collins F., Doumbo O.K., Greenwood B., Hall B.F., Levine M.M., Mendis K., Newman R.D., Plowe C.V., Rodríguez M.H., Sinden R., Slutsker L., Tanner M. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. - PMC - PubMed
-
- Altizer S., Nunn C.L., Lindenfors P. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 2007;76:304–314. - PubMed
-
- Altizer S., Pedersen A.B. Host-pathogen evolution, biodiversity and disease risks for natural populations. In: Carroll S.P., Fox C.W., editors. Conservation Biology: Evolution in Action. Oxford University Press; Oxford: 2008. pp. 259–277.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

