A pilot trial on the treatment of gastroesophageal reflux-related cough in infants
- PMID: 26835260
- PMCID: PMC4728850
- DOI: 10.3978/j.issn.2224-4336.2012.03.03
A pilot trial on the treatment of gastroesophageal reflux-related cough in infants
Abstract
Background: Diagnosing asthma in infancy is largely made on the basis of the symptoms of cough and wheeze. A similar presentation can be seen in neurologically normal infants with excessive gastroesophageal reflux (GER). There are no randomized placebo controlled studies in infants using proton pump inhibitors (PPI) alone or in addition to prokinetic agents.
Objectives: The primary objective was to confirm the presence of excessive GER in a population of infants that also had respiratory symptoms suggestive of asthma. Second, in a randomized placebo-controlled fashion, we determined whether treatment of GER with bethanacol and omeprazole could improve these respiratory symptoms.
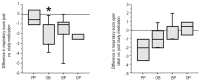
Methods: Infants (n=22) with a history of chronic cough and wheeze were enrolled, if they had evidence of GER by history and an abnormal pH probe or gastric emptying scan. Infants were randomly allocated to four treatment groups: placebo/placebo (PP), omeprazole plus bethanacol (OB), omeprazole/placebo (OP), bethanacol/placebo (BP). Evaluations by clinic questionnaire and exam, home diary, and pH probe data were done before, after study-medication and after open label of OB.
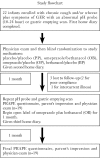
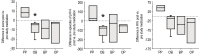
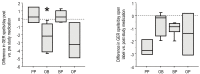
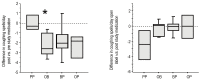
Results: Nineteen children were studied. PP did not affect GER or respiratory symptoms, and did not decrease GER measured by pH probe. In contrast, OB decreased GER as measured by pH probe indices and parental assessment. In association, OB significantly decreased daytime coughing and improved respiratory scores. No adverse effects were reported.
Conclusions: In infants with a clinical presentation suggestive of chronic GER-related cough, the use of omeprazole and bethanacol appears to be viable therapeutic option.
Keywords: Cough; esophagus; gastroesophageal reflux; pediatric asthma.
Conflict of interest statement
Figures
Similar articles
-
[A pilot trial on the treatment of gastroesophageal reflux-related cough in infants].Zhongguo Dang Dai Er Ke Za Zhi. 2012 May;14(5):321-7. Zhongguo Dang Dai Er Ke Za Zhi. 2012. PMID: 22613099 Clinical Trial. Chinese.
-
Gastroesophageal reflux in asthmatics: A double-blind, placebo-controlled crossover study with omeprazole.Chest. 1999 Nov;116(5):1257-64. doi: 10.1378/chest.116.5.1257. Chest. 1999. PMID: 10559084 Clinical Trial.
-
Gastroesophageal reflux in childhood.Curr Probl Surg. 1996 Jan;33(1):1-70. Curr Probl Surg. 1996. PMID: 8536488 Review.
-
Cough and gastroesophageal reflux: from the gastroenterologist end.Pulm Pharmacol Ther. 2009 Apr;22(2):135-8. doi: 10.1016/j.pupt.2008.11.007. Epub 2008 Nov 27. Pulm Pharmacol Ther. 2009. PMID: 19063984 Review.
-
[Effects of proton pump inhibitor on airway hyperresponsiveness in asthmatics with gastroesophageal reflux].Arerugi. 2006 Jun;55(6):641-6. Arerugi. 2006. PMID: 16883100 Japanese.
Cited by
-
Chronic Cough and Gastroesophageal Reflux in Children: CHEST Guideline and Expert Panel Report.Chest. 2019 Jul;156(1):131-140. doi: 10.1016/j.chest.2019.03.035. Epub 2019 Apr 16. Chest. 2019. PMID: 31002783 Free PMC article.
-
Current therapies for gastro-oesophageal reflux in the setting of chronic lung disease: state of the art review.ERJ Open Res. 2020 Nov 10;6(4):00190-2019. doi: 10.1183/23120541.00190-2019. eCollection 2020 Oct. ERJ Open Res. 2020. PMID: 33693049 Free PMC article. Review.
References
-
- Loughlin GM. Respiratory consequences of dysfunctional swallowing and aspiration. Dysphagia 1989;3:126-30. - PubMed
-
- Irwin RS, Madison JM, Fraire AE. The cough reflex and its relation to gastroesophageal reflux. Am J Med 2000;108:73S-8S. - PubMed
-
- Orenstein SR, Shalaby TM, Kelsey SF, et al. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. Am J Gastroenterol 2006;101:628-40. - PubMed
-
- Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32:S1-31. - PubMed
-
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
