Down syndrome and leukemia: insights into leukemogenesis and translational targets
- PMID: 26835364
- PMCID: PMC4729084
- DOI: 10.3978/j.issn.2224-4336.2015.03.03
Down syndrome and leukemia: insights into leukemogenesis and translational targets
Abstract
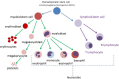
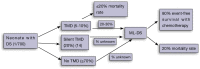
Children with Down syndrome (DS) have a significantly increased risk of childhood leukemia, in particular acute megakaryoblastic leukemia (AMKL) and acute lymphoblastic leukemia (DS-ALL). A pre-leukemia, called transient myeloproliferative disorder (TMD), characterised by a GATA binding protein 1 (GATA1) mutation, affects up to 30% of newborns with DS. In most cases, the pre-leukemia regresses spontaneously, however one-quarter of these children will go on to develop AMKL or myelodysplastic syndrome (MDS) . AMKL and MDS occurring in young children with DS and a GATA1 somatic mutation are collectively termed myeloid leukemia of Down syndrome (ML-DS). This model represents an important multi-step process of leukemogenesis, and further study is required to identify therapeutic targets to potentially prevent development of leukemia. DS-ALL is a high-risk leukemia and mutations in the JAK-STAT pathway are frequently observed. JAK inhibitors may improve outcome for this type of leukemia. Genetic and epigenetic studies have revealed likely candidate drivers involved in development of ML-DS and DS-ALL. Overall this review aims to identify potential impacts of new research on how we manage children with DS, pre-leukemia and leukemia.
Keywords: (3-5)-MeSH headings; Down syndrome (DS); children; leukemia; preleukemia; transient myeloproliferative disorder (TMD) of Down syndrome.
Conflict of interest statement
Figures

References
-
- Hitzler JK, Zipursky A. Origins of leukaemia in children with Down syndrome. Nat Rev Cancer 2005;5:11-20. - PubMed
-
- Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet 2000;355:165-9. - PubMed
-
- NSW Mothers and Babies 2010. Sydney: Centre for Epidemiology and Evidence, NSW Ministry of Health, 2012.
-
- Hasle H, Niemeyer CM, Chessells JM, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia 2003;17:277-82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
