Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity
- PMID: 26835461
- PMCID: PMC4718982
- DOI: 10.3389/fvets.2016.00002
Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity
Abstract
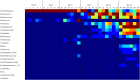
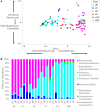
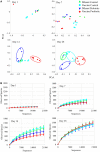
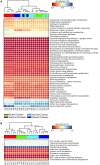
The concept of improving animal health through improved gut health has existed in food animal production for decades; however, only recently have we had the tools to identify microbes in the intestine associated with improved performance. Currently, little is known about how the avian microbiome develops or the factors that affect its composition. To begin to address this knowledge gap, the present study assessed the development of the cecal microbiome in chicks from hatch to 28 days of age with and without a live Salmonella vaccine and/or probiotic supplement; both are products intended to promote gut health. The microbiome of growing chicks develops rapidly from days 1-3, and the microbiome is primarily Enterobacteriaceae, but Firmicutes increase in abundance and taxonomic diversity starting around day 7. As the microbiome continues to develop, the influence of the treatments becomes stronger. Predicted metagenomic content suggests that, functionally, treatment may stimulate more differences at day 14, despite the strong taxonomic differences at day 28. These results demonstrate that these live microbial treatments do impact the development of the bacterial taxa found in the growing chicks; however, additional experiments are needed to understand the biochemical and functional consequences of these alterations.
Keywords: Salmonella; chicken; gut development; microbiome development; probiotic.
Figures
References
-
- Roos NM, de, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr (2000) 71:405–11. - PubMed
-
- Marangon S, Busani L. The use of vaccination in poultry production. Rev Sci Tech (2007) 26:265–74. - PubMed
-
- Saif YM. Diseases of Poultry. Hoboken, NJ: Wiley; (2003).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

