Iterative design of emetine-based prodrug targeting fibroblast activation protein (FAP) and dipeptidyl peptidase IV DPPIV using a tandem enzymatic activation strategy
- PMID: 26835873
- PMCID: PMC6432629
- DOI: 10.1002/pros.23162
Iterative design of emetine-based prodrug targeting fibroblast activation protein (FAP) and dipeptidyl peptidase IV DPPIV using a tandem enzymatic activation strategy
Abstract
Background: There is an urgent need to develop new agents for treating metastatic prostate cancer to overcome multiple drug resistance to the current standard targeted cancer therapy. Emetine is a highly cytotoxic natural product protein synthesis inhibitor, which is toxic to all cell types. Its cytotoxicity can be blocked by derivatizing its N-2' position. Thus emetine can be selectively delivered to cancer cells in the region of metastatic cancer as a prodrug that will be activated by an enzyme selectively overexpressed within the metastatic tumor microenvironment. In this work, we convert emetine to a prodrug activatable by the fibroblast activation protein (FAP), a serine protease overexpressed by the carcinoma associated fibroblasts.
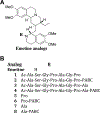
Method: By using an iterative structure-activity relationship strategy, several peptidyl emetine prodrug analogs (1-11) were synthesized by chemical derivatization of emetine at its N-2' position and tested for in-vitro activation by FAP. The lead prodrug 11 is made up of a DPPIV activatable prodrug precursor 10 (Ala-Pro-PABC-Emetine) coupled to FAP substrate (Ala-Ser-Gly-Pro-Ala-Gly-Pro). Activation assays of the prodrugs were performed in purified FAP, DPPIV, FBS, and human serum and were analyzed by LCMS. In vitro cytotoxicity assays of these prodrugs are carried out in prostate (LNCaP, PC3) and breast (MCF7 and MDA-MB-231) cancer cell lines. The prodrugs are also tested in normal immortalized human prostatic epithelial cell line (PrEC).
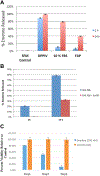
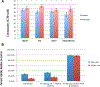
Results: The lead FAP activated emetine prodrug 11 is activated to emetine in tandem by FAP and DPPIV in about 70% conversion within 24 hr. In prostate and breast cancer cell lines treated with prodrug 11, it is found to be equipotent with emetine in the presence of FAP and DPPIV. However, in the PrEC cell line grown in serum free media, prodrug 11 is more than 200-fold less cytotoxic than emetine in the absence of FAP and DPPIV.
Conclusion: This FAP activated prodrug of cytotoxic agent emetine further shows the crucial role of the N-2' position of emetine in controlling its cytotoxicity. Significantly reduced toxicity observed in the PrEC cell line in the absence of FAP and DPPIV shows that prodrug 11 could be systemically delivered to regions of metastatic prostate cancer or other solid tumor for activation by cancer selective enzymes within the cancer microenvironment, such as FAP that is overexpressed by the carcinoma-associated fibroblasts. The two-step tandem enzymatic activation of prodrug 11 by FAP and DPPIV is a strategy for overcoming steric hindrance.
Keywords: dipeptidyl peptidase IV (DPPIV); emetine; fibroblast activation protein (FAP); linker; para-aminobenzyloxycarbonyl (PABC); prodrug.
© 2016 Wiley Periodicals, Inc.
Figures


Similar articles
-
Anticancer activities of emetine prodrugs that are proteolytically activated by the prostate specific antigen (PSA) and evaluation of in vivo toxicity of emetine derivatives.Bioorg Med Chem. 2017 Dec 15;25(24):6707-6717. doi: 10.1016/j.bmc.2017.11.015. Epub 2017 Nov 10. Bioorg Med Chem. 2017. PMID: 29153549 Free PMC article.
-
Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug.J Natl Cancer Inst. 2012 Sep 5;104(17):1320-34. doi: 10.1093/jnci/djs336. Epub 2012 Aug 21. J Natl Cancer Inst. 2012. PMID: 22911669 Free PMC article.
-
Pharmacokinetics and toxicology of a fibroblast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer.Prostate. 2014 Sep;74(13):1308-19. doi: 10.1002/pros.22847. Epub 2014 Jul 22. Prostate. 2014. PMID: 25053236 Free PMC article.
-
Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications.Biochim Biophys Acta Proteins Proteom. 2020 Jul;1868(7):140409. doi: 10.1016/j.bbapap.2020.140409. Epub 2020 Apr 6. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 32171757 Review.
-
Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy.Mol Cancer Ther. 2012 Feb;11(2):257-66. doi: 10.1158/1535-7163.MCT-11-0340. Mol Cancer Ther. 2012. PMID: 22323494 Free PMC article. Review.
Cited by
-
Anticancer activities of emetine prodrugs that are proteolytically activated by the prostate specific antigen (PSA) and evaluation of in vivo toxicity of emetine derivatives.Bioorg Med Chem. 2017 Dec 15;25(24):6707-6717. doi: 10.1016/j.bmc.2017.11.015. Epub 2017 Nov 10. Bioorg Med Chem. 2017. PMID: 29153549 Free PMC article.
-
Tumor-targeting efficacy of a BF211 prodrug through hydrolysis by fibroblast activation protein-α.Acta Pharmacol Sin. 2018 Mar;39(3):415-424. doi: 10.1038/aps.2017.121. Epub 2017 Nov 9. Acta Pharmacol Sin. 2018. PMID: 29119969 Free PMC article.
-
Targeting of activated fibroblasts for imaging and therapy.EJNMMI Radiopharm Chem. 2019 Jul 25;4(1):16. doi: 10.1186/s41181-019-0069-0. EJNMMI Radiopharm Chem. 2019. PMID: 31659499 Free PMC article. Review.
-
Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma.Tumour Biol. 2016 Oct;37(10):13961-13971. doi: 10.1007/s13277-016-5274-9. Epub 2016 Aug 4. Tumour Biol. 2016. PMID: 27492457
-
Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome: Current Therapeutic Options and Potential Targets for Novel Therapies.Drugs. 2017 Dec;77(18):1935-1966. doi: 10.1007/s40265-017-0830-1. Drugs. 2017. PMID: 29143192 Free PMC article. Review.
References
-
- Wiegrebe W, Kramer WJ, Shamma M. The emetine alkaloids. J Nat Prod 1984;47:397–408.
-
- Jimenez A, Carrasco L, Vazquez D. Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors. Biochemistry 1977;16(21):4727–4730. - PubMed
-
- Akinboye ES, Bakare O. Biological activities of emetine. Open Nat Prod J 2011;4:8–15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

