Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer
- PMID: 26835979
- PMCID: PMC4851343
- DOI: 10.1021/acsnano.5b07781
Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer
Abstract
Urgent intervention is required to improve the 5 year survival rate of pancreatic ductal adenocarcinoma (PDAC). While the four-drug regimen, FOLFIRINOX (comprising irinotecan, 5-fluorouracil, oxaliplatin, and leucovorin), has a better survival outcome than the more frequently used gemcitabine, the former treatment platform is highly toxic and restricted for use in patients with good performance status. Since irinotecan contributes significantly to FOLFIRINOX toxicity (bone marrow and gastrointestinal tract), our aim was to reduce the toxicity of this drug by a custom-designed mesoporous silica nanoparticle (MSNP) platform, which uses a proton gradient for high-dose irinotecan loading across a coated lipid bilayer (LB). The improved stability of the LB-coated MSNP (LB-MSNP) carrier allowed less drug leakage systemically with increased drug concentrations at the tumor sites of an orthotopic Kras-derived PDAC model compared to liposomes. The LB-MSNP nanocarrier was also more efficient for treating tumor metastases. Equally important, the reduced leakage and slower rate of drug release by the LB-MSNP carrier dramatically reduced the rate of bone marrow, gastrointestinal, and liver toxicity compared to the liposomal carrier. We propose that the combination of high efficacy and reduced toxicity by the LB-MSNP carrier could facilitate the use of irinotecan as a first-line therapeutic to improve PDAC survival.
Keywords: FOLFIRINOX; irinotecan; lipid bilayer; mesoporous silica nanoparticle; pancreatic cancer; proton gradient; toxicity reduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
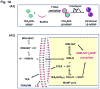






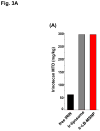









References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA-Cancer J Clin. 2014;64:9–29. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. New Engl J Med. 2011;364:1817–1825. - PubMed
-
- Ueno H, Okusaka T, Funakoshi A, Ishii H, Yamao K, Ishikawa O, Ohkawa S, Saitoh S. A Phase II Study of Weekly Irinotecan as First-Line Therapy for Patients with Metastatic Pancreatic Cancer. Cancer Chemoth Pharm. 2007;59:447–454. - PubMed
-
- Chou TH, Chen SC, Chu IM. Effect of Composition on the Stability of Liposomal Irinotecan Prepared by a pH Gradient Method. J Biosci Bioeng. 2003;95:405–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

