How Will Evolving Future Therapies and Strategies Change How We Position the Use of Biologics in Moderate to Severely Active Inflammatory Bowel Disease
- PMID: 26835982
- PMCID: PMC5953904
- DOI: 10.1097/MIB.0000000000000661
How Will Evolving Future Therapies and Strategies Change How We Position the Use of Biologics in Moderate to Severely Active Inflammatory Bowel Disease
Abstract
Several biological agents have been added to our armamentarium of treatment options for moderate to severely active inflammatory bowel diseases, and this number is expected to only increase in the near future. With our growing understanding of disease mechanisms and pharmacokinetics, we are now able to target several mechanisms of action to achieve key endpoints (steroid-free remission and mucosal healing) associated with improved long-term disease-related outcomes. In this context, concerns arise regarding the optimal positioning of currently available biologics and key biologics in development. In this review, we will discuss the currently available evidence for comparative effectiveness of biological agents approved for the use in moderate to severely active inflammatory bowel diseases, with a focus on practical considerations to be made when using these agents in practice. We will further review novel biological agents and small molecule inhibitors in development and discuss future opportunities through which providers may personalize treatment decisions to achieve optimal treatment outcomes.
Conflict of interest statement
Figures
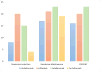
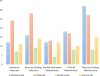
References
-
- Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–19. - PubMed
-
- Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. - PubMed
-
- Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246–56.e6. - PubMed
-
- Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014;63:88–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

